Search Thermo Fisher Scientific
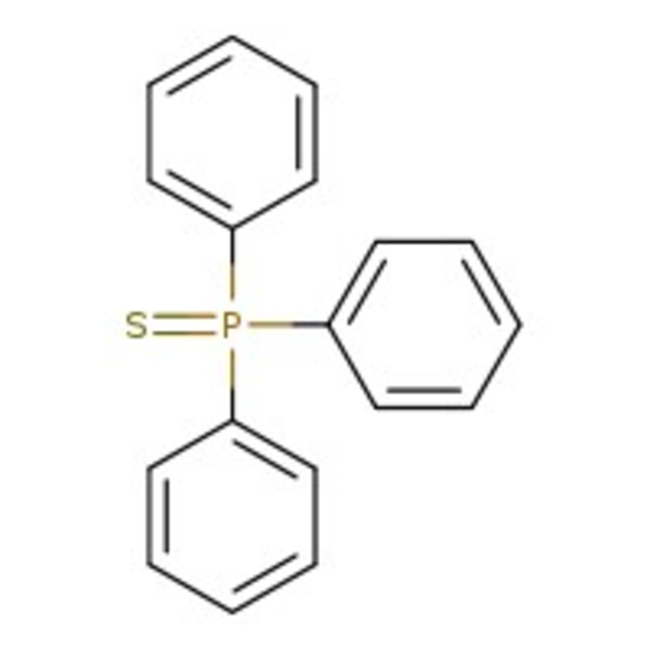
Triphenylphosphine sulfide, 98%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA16884.14 | 25 g |
Catalog number ALFA16884.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialTriphenylphosphine sulfide
CAS3878-45-3
Health Hazard 1Danger
Health Hazard 2GHS H Statement
H331-H302-H312-H315-H319-H335
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H331-H302-H312-H315-H319-H335
Toxic if inhaled.
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3GHS P Statement
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
Triphenylphosphine sulfide involves in the , conversion of epoxides to episulfides (thiiranes) in presence of TFA of the same stereochemistry. It acts as a ligand for the Pd-catalyzed bisalkoxycarbonylation of olefins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triphenylphosphine sulfide involves in the , conversion of epoxides to episulfides (thiiranes) in presence of TFA of the same stereochemistry. It acts as a ligand for the Pd-catalyzed bisalkoxycarbonylation of olefins.
Solubility
Insoluble in water.
Notes
Store in a cool, dry, conditions in well sealed container. Store away from oxidizing agents.
Triphenylphosphine sulfide involves in the , conversion of epoxides to episulfides (thiiranes) in presence of TFA of the same stereochemistry. It acts as a ligand for the Pd-catalyzed bisalkoxycarbonylation of olefins.
Solubility
Insoluble in water.
Notes
Store in a cool, dry, conditions in well sealed container. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- W. W. Schweikert; Edward A. Meyers. Crystal structure of the triphenylphosphine sulfide-iodine addition complex. J. Phys. Chem.1968, 72 (5), 1561-1565.
- B. Ziemer; A. Rabis and H.-U. Steinberger. Triclinic polymorphs of triphenylphosphine and triphenylphosphine sulfide. Acta Cryst.2000, C56 e58-e59.
- In the presence of TFA, converts epoxides to episulfides (thiiranes) of the same stereochemistry: J. Am. Chem. Soc., 94, 2880 (1972).
- The Pd-catalyzed bisalkoxycarbonylation of olefins gives improved yields in the presence of triphenylphosphine sulfide as a ligand. The phosphine oxide and selenide were significantly less effective, and no product was obtained with triphenylphosphine itself: Tetrahedron Lett, 39, 7529 (1998):