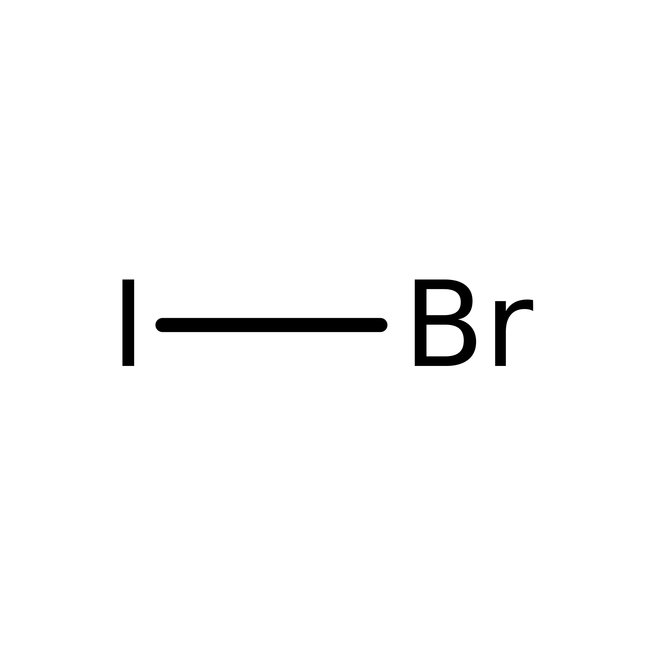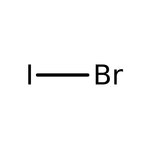Search Thermo Fisher Scientific
Thermo Scientific Chemicals
Iodine monobromide, 98%, Thermo Scientific Chemicals
CAS: 7789-33-5 | BrI | 206.81 g/mol
Catalog number ALFA16158.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS55804-65-4
IUPAC Name4-oxo-3-oxa-13-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1,5,7,9(17)-tetraene-5-carboxylate
Molecular FormulaC16H14NO4
InChI KeyKCDCNGXPPGQERR-UHFFFAOYSA-M
SMILES[O-]C(=O)C1=CC2=CC3=C4N(CCC3)CCCC4=C2OC1=O
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
UV Lambda max409 nm (in Basic Ethanol)
Molar absorption coefficient19900 l/(mol.cm) (at Lambda max)
Fluorescence max470 nm (in Basic Ethanol)
Appearance (Form)Crystalline powder
View more
Iodine monobromide is used as an electrophile employed in a new synthetic approach to polyketide structural units. It is also used in iodometry and serves as a source of I+. It is a powerful iodinating agent used in organic synthesis as well as involved in the synthesis of radioiodinated fatty acids for heart imaging.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Iodine monobromide is used as an electrophile employed in a new synthetic approach to polyketide structural units. It is also used in iodometry and serves as a source of I+. It is a powerful iodinating agent used in organic synthesis as well as involved in the synthesis of radioiodinated fatty acids for heart imaging.
Solubility
Soluble in water, alcohol, ether, carbon disulfide and glacial acetic acid.
Notes
Air, light and moisture sensitive. Store in cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, phosphorus, sodium and sodiumoxides and potassium.
Iodine monobromide is used as an electrophile employed in a new synthetic approach to polyketide structural units. It is also used in iodometry and serves as a source of I+. It is a powerful iodinating agent used in organic synthesis as well as involved in the synthesis of radioiodinated fatty acids for heart imaging.
Solubility
Soluble in water, alcohol, ether, carbon disulfide and glacial acetic acid.
Notes
Air, light and moisture sensitive. Store in cool place. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, phosphorus, sodium and sodiumoxides and potassium.
RUO – Research Use Only
General References:
- Selective brominating agent, considered to be intermediate in electrophilic power between bromine and iodine: J. Chem. Soc., 1509 (1939); J. Am. Chem. Soc., 60, 256 (1938); Chem. Commun., 849 (1968).
- Also found to give higher diastereoselectivity than iodine in the cyclization of certain homoallylic carbonates: Tetrahedron Lett., 33, 6439 (1992).
- Reagent for mild, selective deprotection of TBDMS ethers in carbohydrates and nucleosides: Synlett, 311 (1999).
- Khalil, A.; Ishita, K.; Ali, T.; Tiwari, R.; Riachy, R.; Toppino, A.; Tjarks, W. Iodine Monochloride Facilitated Deglycosylation, Anomerization, and Isomerization of 3-Substituted Thymidine Analogues. Nucleosides Nucleotides Nucleic Acids 2014, 33 (12), 786-799.
- Janas, D.; Herman, A. P.; Boncel, S.; Koziol, K. K. Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires. Carbon 2014, 73, 225-233.