Search Thermo Fisher Scientific
Thermo Scientific Chemicals
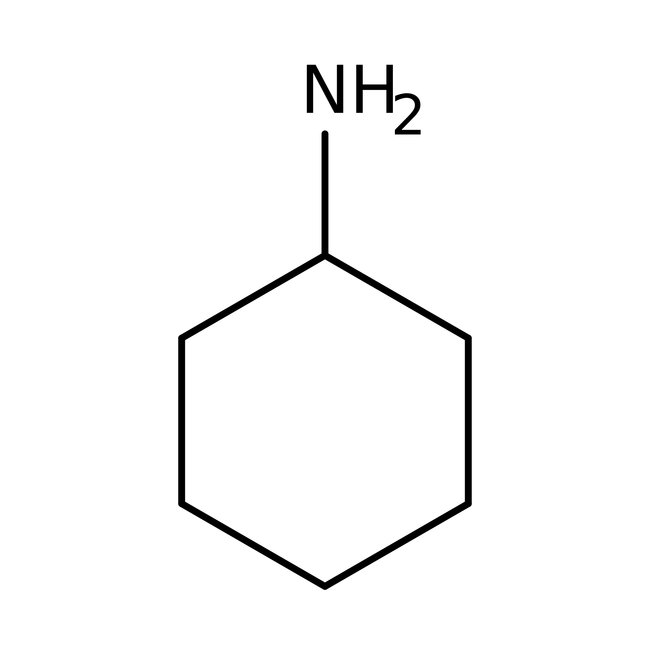
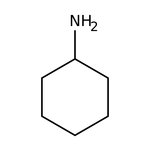
Cyclohexylamine, 98+%, Thermo Scientific Chemicals
CAS: 108-91-8 | C6H13N | 99.177 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA15851.AP | 500 mL |
Catalog number ALFA15851.AP
Price (MYR)
317.00
Quantity:
500 mL
Price (MYR)
317.00
Specifications
Chemical Name or MaterialCyclohexylamine
CAS108-91-8
Health Hazard 1H226-H301+H311-H314-H361f
Health Hazard 2GHS H Statement
H314-H226-H361-H302-H312
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Suspected of damaging fertility or the unborn child.
Harmful if swallowed.
Harmful in contact with skin.
H314-H226-H361-H302-H312
Causes severe skin burns and eye damage.
Flammable liquid and vapour.
Suspected of damaging fertility or the unborn child.
Harmful if swallowed.
Harmful in contact with skin.
Health Hazard 3P201-P202-P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P281-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Cyclohexylamine is a useful as an intermediate in organic synthesis. It acts as a precursor to prepare pendant-armed dialdehydes, bromohexine HCl and sulfenamide-based reagents. It is widely used in the production of vulcanization accelerators like N-cyclohexyl-2-benzothiazole sulfonamide (CBS) and artificial sweeteners (cyclamates). As a corrosion inhibitor, it is used in the treatment of boiler water and prevents corrosion of metal surfaces that arise due to dissolved carbon dioxide. It is involved as a solvent in the synthesis of an inorganic-organic hybrid semiconductor, ZnS/CHA (CHA = cyclohexylamine) nanocomposites.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cyclohexylamine is a useful as an intermediate in organic synthesis. It acts as a precursor to prepare pendant-armed dialdehydes, bromohexine HCl and sulfenamide-based reagents. It is widely used in the production of vulcanization accelerators like N-cyclohexyl-2-benzothiazole sulfonamide (CBS) and artificial sweeteners (cyclamates). As a corrosion inhibitor, it is used in the treatment of boiler water and prevents corrosion of metal surfaces that arise due to dissolved carbon dioxide. It is involved as a solvent in the synthesis of an inorganic-organic hybrid semiconductor, ZnS/CHA (CHA = cyclohexylamine) nanocomposites.
Solubility
Miscible with water, ethanol, ethers, acetone, alcohol and ketones.
Notes
Air and moisture sensitive. Incompatible with strong oxidizing agents, carbon dioxide, sodium hypochlorite, organic acids, mineral acids and peroxides.
Cyclohexylamine is a useful as an intermediate in organic synthesis. It acts as a precursor to prepare pendant-armed dialdehydes, bromohexine HCl and sulfenamide-based reagents. It is widely used in the production of vulcanization accelerators like N-cyclohexyl-2-benzothiazole sulfonamide (CBS) and artificial sweeteners (cyclamates). As a corrosion inhibitor, it is used in the treatment of boiler water and prevents corrosion of metal surfaces that arise due to dissolved carbon dioxide. It is involved as a solvent in the synthesis of an inorganic-organic hybrid semiconductor, ZnS/CHA (CHA = cyclohexylamine) nanocomposites.
Solubility
Miscible with water, ethanol, ethers, acetone, alcohol and ketones.
Notes
Air and moisture sensitive. Incompatible with strong oxidizing agents, carbon dioxide, sodium hypochlorite, organic acids, mineral acids and peroxides.
RUO – Research Use Only
General References:
- For protection of an aldehyde as its cyclohexyl imine, see, e.g.: Org. Synth. Coll., 8, 586 (1993). Cyclohexyl imines of aldehydes have found use in the directed aldol synthesis: reaction with a less reactive ketone takes place in preference to self-condensation of the aldehyde. See, e.g.: Org. Synth. Coll., 6, 901 (1988):
- Darkhalil, I. D.; Klaassen, J. J.; Deodhar, B. S.; Gounev, T. K.; Durig, J. R. Conformational stability, infrared and Raman spectra, vibrational assignments, and theoretical calculations of cyclohexylamine. J. Mol. Struct. 2015, 1088, 169-178.
- Horvath, B.; Kaszonyi, A.; Rakottyay, K.; Vajicek, S.; Lepesova, L.; Seemann, L.; Stolcova, M. Towards the mechanism of the oxidation of cyclohexylamine by molecular oxygen over alumina-based catalysts. React. Kinet. Mech. Catal 2015, 115 (1), 231-250.