Search Thermo Fisher Scientific
Thermo Scientific Chemicals
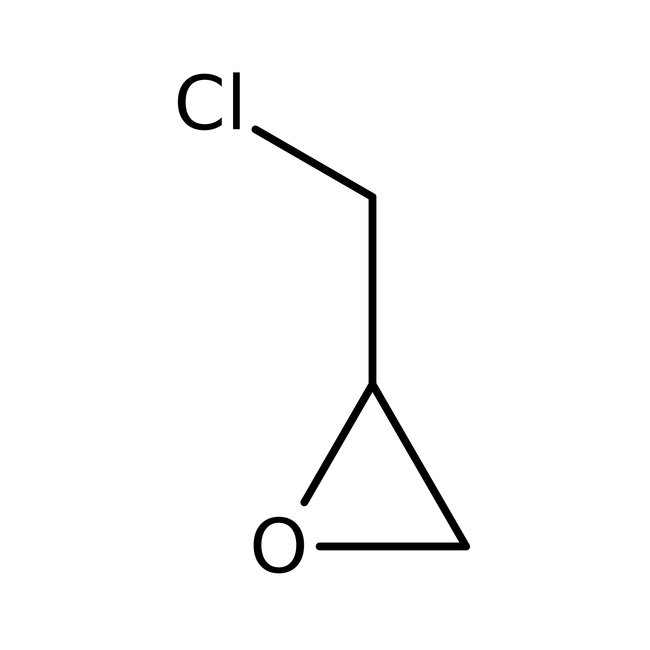
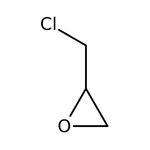
(±)-Epichlorohydrin, 99%, Thermo Scientific Chemicals
CAS: 106-89-8 | C3H5ClO | 92.522 g/mol
Catalog number ALFA15823.0E
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 g
Chemical Identifiers
CAS553-97-9
Specifications Specification Sheet
Specification Sheet
FormCrystalline powder
Identification (FTIR)Conforms
Proton NMRConforms
Appearance (Color)Yellow to brown
Assay (GC)≥97.5%
Epichlorohydrin is used in the manufacture of polyamines and polyquaternary ammonium salts, as flocculents in water and wastewater treatment. It is used in the synthesis of surface active agents for washing products and toiletries. Polyamine-epichlorohydrin resin is used in paper industry to improve paper wet-strength. It acts as a chemical intermediate and widely used in the manufacture of epoxy resins, epichlorohydrin elastomers and polyamide-epichlorohydrin resins.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Epichlorohydrin is used in the manufacture of polyamines and polyquaternary ammonium salts, as flocculents in water and wastewater treatment. It is used in the synthesis of surface active agents for washing products and toiletries. Polyamine-epichlorohydrin resin is used in paper industry to improve paper wet-strength. It acts as a chemical intermediate and widely used in the manufacture of epoxy resins, epichlorohydrin elastomers and polyamide-epichlorohydrin resins.
Solubility
Miscible with water and organic polar solvents.
Notes
Keep away from sources of ignition. Keep container tightly closed in a dry and well-ventilated place.
Epichlorohydrin is used in the manufacture of polyamines and polyquaternary ammonium salts, as flocculents in water and wastewater treatment. It is used in the synthesis of surface active agents for washing products and toiletries. Polyamine-epichlorohydrin resin is used in paper industry to improve paper wet-strength. It acts as a chemical intermediate and widely used in the manufacture of epoxy resins, epichlorohydrin elastomers and polyamide-epichlorohydrin resins.
Solubility
Miscible with water and organic polar solvents.
Notes
Keep away from sources of ignition. Keep container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- For use as an acid scavenger in bromination reactions, see: J. Am. Chem. Soc., 95, 4419 (1973).
- The preparation of glycidyl ethers, often carried out in two steps, via the ring-opened chlorohydrin ether and subsequent ring-closure with base, can be achieved as a single step, under phase-transfer conditions: Synthesis, 117 (1983). For extension to diglycidyl ethers of diols, see: Synthesis, 649 (1985).
- Reaction with primary amines provides a one-step route to 3-azetidinols: J. Org. Chem., 55, 2920 (1990):
- Zhang, Y.; Tan, Z.; Liu, B.; Mao, D.; Xiong, C. Coconut shell activated carbon tethered ionic liquids for continuous cycloaddition of CO2 to epichlorohydrin in packed bed reactor. Catal. Commun. 2015, 68, 73-76.
- Xiao, S.; Zhang, C.; Chen, R.; Chen, F. Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe3O4 microspheres. New. J. Chem. 2015, 39 (6), 4924-4932.