Search Thermo Fisher Scientific
Thermo Scientific Chemicals
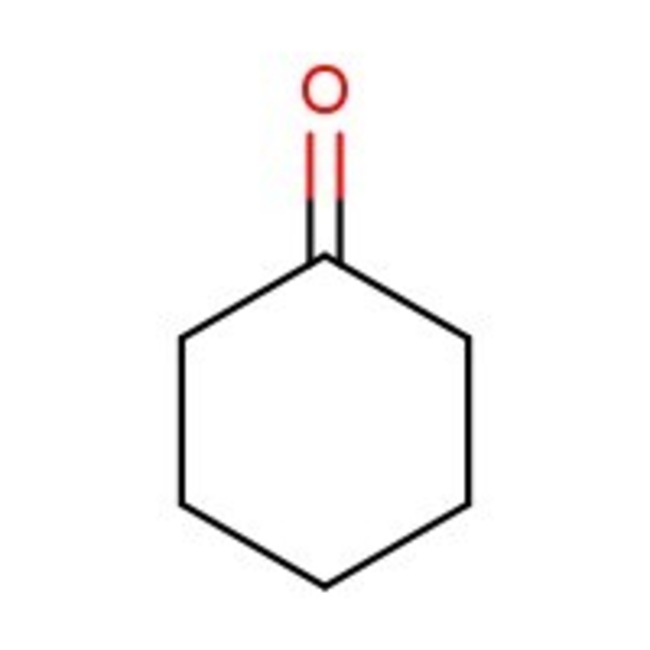
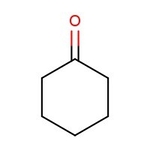
Cyclohexanone, 99+%, Thermo Scientific Chemicals
Catalog number ALFA15607.0F
Price (MYR)
571.00
Quantity:
2500 mL
Price (MYR)
571.00
Specifications
Chemical Name or MaterialCyclohexanone
Melting Point°C to 26°C
CAS108-94-1
Health Hazard 1H226-H302+H312+H332-H315-H318-H335-H336
Health Hazard 2GHS H Statement
H226-H332
H226-H332
View more
Cyclohexanone is used in the preparation of synthetic polymer such as nylon 6,6 and nylon 6. It acts as a solvent in organic synthesis. Its derivatives are used for the synthesis of dyes, rubber chemicals, plasticizers and pharmaceuticals. Further, it is involved in the preparation of cyclohexanone oxime, which rearranges to give caprolactam in the presence of sulfuric acid catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cyclohexanone is used in the preparation of synthetic polymer such as nylon 6,6 and nylon 6. It acts as a solvent in organic synthesis. Its derivatives are used for the synthesis of dyes, rubber chemicals, plasticizers and pharmaceuticals. Further, it is involved in the preparation of cyclohexanone oxime, which rearranges to give caprolactam in the presence of sulfuric acid catalyst.
Solubility
Miscible with diethyl ether, acetone and alcohol. Slightly miscible with water.
Notes
Incompatible with strong oxidizing agents, red metals, lead, amines, sulfuric acid, nitric acid, strong acids, aliphatic amines, rubber, resins and alkalies.
Cyclohexanone is used in the preparation of synthetic polymer such as nylon 6,6 and nylon 6. It acts as a solvent in organic synthesis. Its derivatives are used for the synthesis of dyes, rubber chemicals, plasticizers and pharmaceuticals. Further, it is involved in the preparation of cyclohexanone oxime, which rearranges to give caprolactam in the presence of sulfuric acid catalyst.
Solubility
Miscible with diethyl ether, acetone and alcohol. Slightly miscible with water.
Notes
Incompatible with strong oxidizing agents, red metals, lead, amines, sulfuric acid, nitric acid, strong acids, aliphatic amines, rubber, resins and alkalies.
RUO – Research Use Only
General References:
- Patil, R. D.; Sasson, Y. Selective transfer hydrogenation of phenol to cyclohexanone on supported palladium catalyst using potassium formate as hydrogen source under open atmosphere. Appl. Catal., A 2015, 499, 227-231.
- Anilkumar, M.; Hoelderich, W. F. A one step synthesis of caprolactam out of cyclohexanone by combinded ammoximation and Beckmann rearrangement over Nb-MCM-41 catalysts. Appl. Catal., B 2015, 165, 87-93.