Search Thermo Fisher Scientific
Thermo Scientific Chemicals
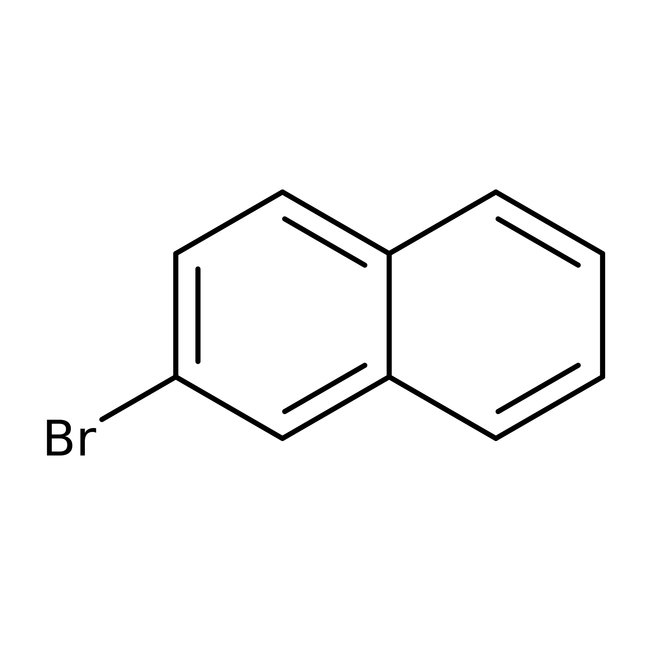
2-Bromonaphthalene, 98+%, Thermo Scientific Chemicals
CAS: 580-13-2 | C10H7Br | 207.07 g/mol
Catalog number ALFA15300.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Chemical Identifiers
CAS16063-69-7
IUPAC Name2,4,6-trichloropyridine
Molecular FormulaC5H2Cl3N
InChI KeyFJNNGKMAGDPVIU-UHFFFAOYSA-N
SMILESClC1=CC(Cl)=NC(Cl)=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to light yellow
Appearance (Form)Crystals or crystalline solid
Infrared spectrumConforms
Melting point31°C to 37°C
GC>=97.5 %
2-Bromonaphthalene is used as a raw material in the preparation of biaryls through Suzuki cross coupling reaction. Further, it plays a vital role in the preparation of dyes. It is also used to study potential tumorigenicity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromonaphthalene is used as a raw material in the preparation of biaryls through Suzuki cross coupling reaction. Further, it plays a vital role in the preparation of dyes. It is also used to study potential tumorigenicity.
Solubility
Slightly soluble in water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
2-Bromonaphthalene is used as a raw material in the preparation of biaryls through Suzuki cross coupling reaction. Further, it plays a vital role in the preparation of dyes. It is also used to study potential tumorigenicity.
Solubility
Slightly soluble in water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- The Grignard reagent has been reacted with N-benzylproline methyl ester in the synthesis of oxazaborolidines (compare (S)-2-Methyl-CBS-oxazaborolidine monohydrate, L09219) for use as chiral auxiliaries: J. Org. Chem., 56, 442 (1991).
- Hopkins, B. A.; Garlets, Z. J.; Wolfe, J. P. Development of Enantioselective Palladium-Catalyzed Alkene Carboalkoxylation Reactions for the Synthesis of Tetrahydrofurans. Angew. Chem. Int. Ed. 2015, 54 (45), 13390-13392
- Xu, L.; Suo, H.; Liang, X.; Wang, L.; Guo, Y.; Jiang, S. Au nanoparticle decorated graphene oxide as a novel coating for solid-phase microextraction. RSC Adv. 2015, 5 (52), 41536-41543.