Search Thermo Fisher Scientific
Thermo Scientific Chemicals
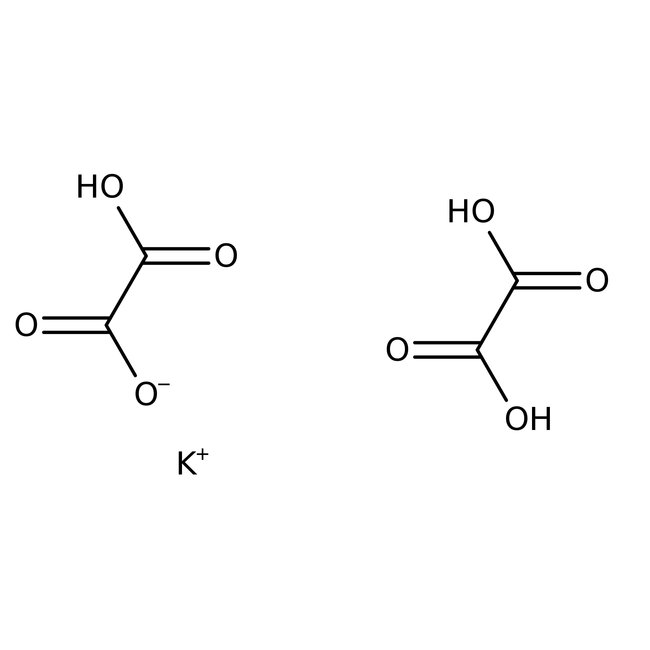
Potassium trihydrogen dioxalate dihydrate, 98+%, Thermo Scientific Chemicals
CAS: 6100-20-5 | C4H3KO8 | 218.16 g/mol
Catalog number ALFA14933.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Chemical Identifiers
CAS95-57-8
IUPAC Name2-chlorophenol
Molecular FormulaC6H5ClO
InChI KeyISPYQTSUDJAMAB-UHFFFAOYSA-N
SMILESOC1=CC=CC=C1Cl
View more
Specifications Specification Sheet
Specification Sheet
FormLiquid
Assay (Silylated GC)≥98.5%
Refractive Index1.5580-1.5610 @ 20?C
Appearance (Color)Clear colorless to pale yellow or pink
Identification (FTIR)Conforms
Potassium trihydrogen dioxalate dihydrat is used in metal polishing determination of pH. And it is also used in photography, marble grinding, and to remove ink stains.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Potassium trihydrogen dioxalate dihydrat is used in metal polishing determination of pH. And it is also used in photography, marble grinding, and to remove ink stains.
Solubility
Soluble in water
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
Potassium trihydrogen dioxalate dihydrat is used in metal polishing determination of pH. And it is also used in photography, marble grinding, and to remove ink stains.
Solubility
Soluble in water
Notes
Store away from strong oxidizing agents. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- T. Honda.; K. Murase.; T. Hirato.; Y. Awakura. pH measurement in the vicinity of a cathode evolving hydrogen gas using an antimony microelectrode. Journal of Applied Electrochemistry. 1998, 28, (6), 617-622.
- Hong Wen.; Tonglei Li.; Kenneth R. Morris.; Kinam Park. How Solvents Affect Acetaminophen Etching Pattern Formation: Interaction between Solvent and Acetaminophen at the Solid/Liquid Interface. J. Phys. Chem. B. 2004, 108, (7), 2270-2278.