Search Thermo Fisher Scientific
Thermo Scientific Chemicals
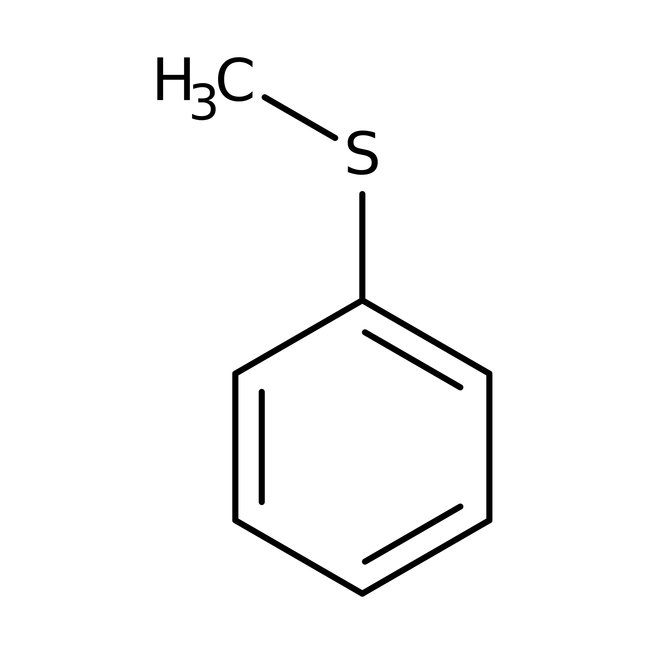
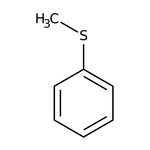
Thioanisole, 99%, Thermo Scientific Chemicals
CAS: 100-68-5 | C7H8S | 124.201 g/mol
Catalog number ALFA14846.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialThioanisole
CAS100-68-5
Health Hazard 1H227-H302-H315-H317-H319
Health Hazard 2GHS H Statement
H226-H302-H315-H319-H335
Flammable liquid and vapour.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H226-H302-H315-H319-H335
Flammable liquid and vapour.
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P261-P264b-P270-P272-P280-P301+P312-P302+P352-P305+P351+P338-P330-P333+P313-P362-P370+P378q-P501c
View more
Thioanisole is used as an intermediate in the preparation of dyes, pharmaceuticals and agrochemicals. It is also used to prepare stable sulfonium salts, sulfoxides and sufones. It is utilized for the cleavage of methyl ethers in association with triflic acid. It acts as a flavoring agent or an adjuvant. It is an important starting material for the synthesis of 3-substituted benzo[b]thiophenes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Thioanisole is used as an intermediate in the preparation of dyes, pharmaceuticals and agrochemicals. It is also used to prepare stable sulfonium salts, sulfoxides and sufones. It is utilized for the cleavage of methyl ethers in association with triflic acid. It acts as a flavoring agent or an adjuvant. It is an important starting material for the synthesis of 3-substituted benzo[b]thiophenes.
Solubility
Miscible with most common organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
Thioanisole is used as an intermediate in the preparation of dyes, pharmaceuticals and agrochemicals. It is also used to prepare stable sulfonium salts, sulfoxides and sufones. It is utilized for the cleavage of methyl ethers in association with triflic acid. It acts as a flavoring agent or an adjuvant. It is an important starting material for the synthesis of 3-substituted benzo[b]thiophenes.
Solubility
Miscible with most common organic solvents. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Monolithiation is thought to occur initially on the ring, but rearrangement then gives the more stable phenylthiomethyllithium: J. Org. Chem., 31, 4097 (1966); Tetrahedron Lett., 1961 (1977). Further metallation occurs at the ortho-position. See, e.g.: Tetrahedron, 46, 861 (1990). Demetallation and acylation is the basis of a route to 3-substituted benzo[b]thiophenes: Synthesis, 888 (1988):
- Cation scavenger in the acidic deprotection of peptides; see, e.g.: Int. J. Pept. Prot. Res., 36, 255 (1990). See Appendix 6. The combination of the soft nucleophile thioanisole and the hard acid triflic acid has been used for the cleavage of methyl ethers, e.g. O-methyltyrosine, resistant to other methods: J. Chem. Soc., Chem. Commun., 971 (1979). The same conditions have been used for the cleavage of Cbz, Boc, benzyl and 4-methoxybenzyl protecting groups in peptide synthesis: Chem. Pharm. Bull., 28, 1214 (1980); 29, 600 (1981); J. Chem. Soc., Chem. Commun., 101 (1980); see also: Angew. Chem. Int. Ed., 33, 1729 (1994).
- Trehoux, A.; Roux, Y.; Guillot, R.; Mahy, J. P.; Avenier, F. Catalytic oxidation of dibenzothiophene and thioanisole by a diiron(III) complex and hydrogen peroxide. J. Mol. Catal. A: Chem. 2015, 396, 40-46.
- Li, S. L.; Xu, X.; Truhlar, D. G. Computational simulation and interpretation of the low-lying excited electronic states and electronic spectrum of thioanisole. Phys. Chem. Chem. Phys. 2015, 17 (31), 20093-20099.