Search Thermo Fisher Scientific
Thermo Scientific Chemicals
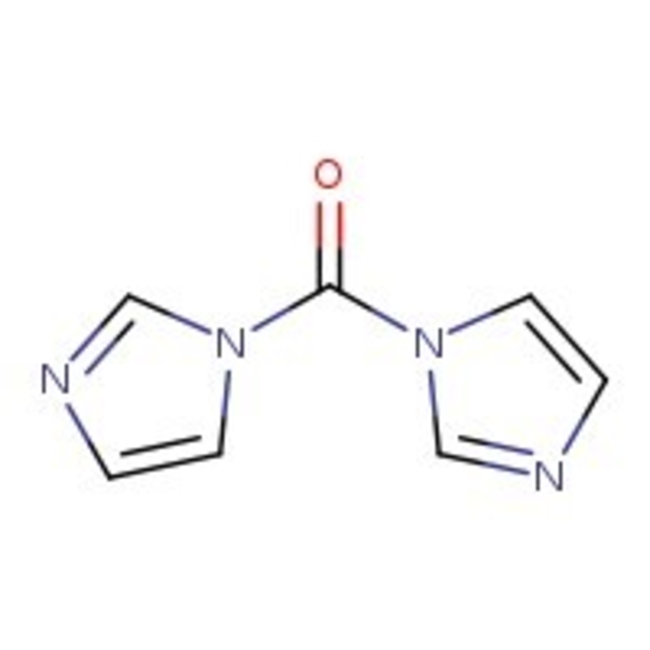
1,1'-Carbonyldiimidazole, 97%, Thermo Scientific Chemicals
CAS: 530-62-1 | C7H6N4O | 162.15 g/mol
Catalog number ALFA14688.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS3483-12-3
IUPAC Name(2R,3R)-1,4-disulfanylbutane-2,3-diol
Molecular FormulaC4H10O2S2
InChI KeyVHJLVAABSRFDPM-IMJSIDKUSA-N
SMILESO[C@@H](CS)[C@@H](O)CS
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Melting point38°C to 43°C
Infrared spectrumConforms
Appearance (Form)Powder or beads
Titration Iodimetric97.5 to 102.5 %
Peptide coupling reagent1,1'-Carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis. It is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Peptide coupling reagent1,1′-Carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis. It is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate.
Solubility
Soluble in dimethylformamide.
Notes
Store in a cool place. Incompatible with water, strong acids, strong oxidizing agents, strong bases and amines.
Peptide coupling reagent1,1′-Carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis. It is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate.
Solubility
Soluble in dimethylformamide.
Notes
Store in a cool place. Incompatible with water, strong acids, strong oxidizing agents, strong bases and amines.
RUO – Research Use Only
General References:
- Reagent for peptide coupling via the acylimidazolide: Liebigs Ann. Chem., 609, 75 (1957); J. Am. Chem. Soc., 80, 4423 (1958); 82, 4596 (1960): J. Org. Chem., 27, 2094 (1962). For peptide reagents, see Appendix 6.
- One-pot esterification of a carboxylic acid with t-BuOH occurs in the presence of DBU: Synthesis, 833 (1982). The reactivity of acyl imidazolides can be increased by N-alkylation: Chem. Pharm. Bull., 32, 5044 (1984).
- Acylimidazolides can also be reduced to aldehydes by DIBAL-H. This reaction has been applied to N-protected amino acids: J. Chem. Soc., Chem. Commun., 79 (1979).
- Dehydrates aldoximes, including chiral oximes, to nitriles in high yield: J. Chem. Soc., Chem. Commun., 628 (1973); Synth. Commun., 12, 25 (1982). Ketoximes can be converted to amides by the spontaneous Beckmann rearrangement of imidazolium salts: Chem. Pharm. Bull., 32, 2560 (1984):
- ß-Hydroxy amino acids are dehydrated to dehydroamino acids: Synthesis, 968 (1982).
- ɑß-Dihydroxyketones are converted toɑ-diketones: Synth. Commun., 23, 2219 (1993).
- Lanzillotto, M.; Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Mechanochemical 1,1'-Carbonyldiimidazole-Mediated Synthesis of Carbamates. ACS Sustainable Chem. Eng. 2015, 3 (11), 2882-2889.
- Rodrigues, M. T.; Santos, M. S.; Santos, H.; Coelho, F. 1, 1'-Carbonyldiimidazole mediates the synthesis of N-substituted imidazole derivatives from Morita-Baylis-Hillman adducts. Tetrahedron Lett. 2014, 55 (1), 180-183.
- Lafrance, D.; Bowles, P.; Leeman, K.; Rafka, R. Mild Decarboxylative Activation of Malonic Acid Derivatives by 1, 1'-Carbonyldiimidazole. Org. Lett. 2011, 13 (9), 2322-2325.