Search Thermo Fisher Scientific
Thermo Scientific Chemicals
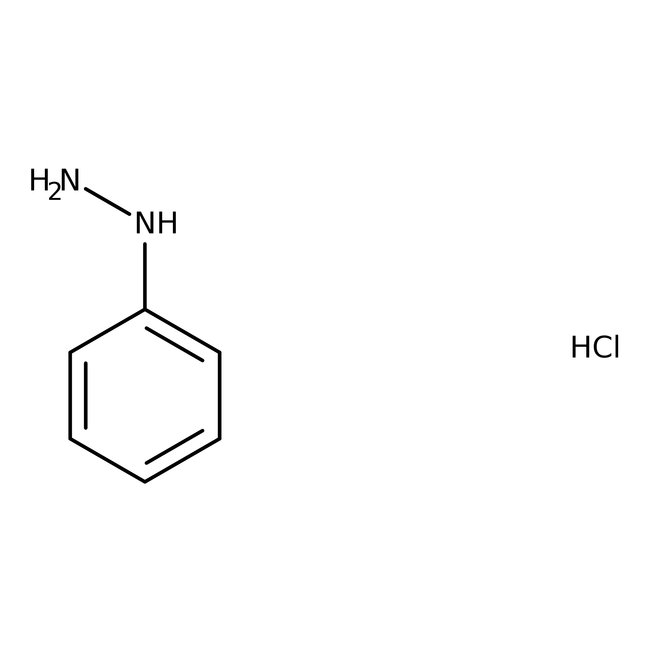
Phenylhydrazine hydrochloride, 99%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA14645.36 | 500 g |
Catalog number ALFA14645.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialPhenylhydrazine hydrochloride
CAS59-88-1
Health Hazard 1H301+H311+H331-H315-H317-H319-H341-H351-H372
Health Hazard 2GHS H Statement
H301-H311-H331-H350-H372-H341-H315-H319-H317
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
May cause cancer.
Causes damage to organs through prolonged or repeated exposure.
Suspected of causing genetic defects.
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
H301-H311-H331-H350-H372-H341-H315-H319-H317
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
May cause cancer.
Causes damage to organs through prolonged or repeated exposure.
Suspected of causing genetic defects.
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
Health Hazard 3P201-P202-P260-P264b-P270-P271-P272-P280-P281-P301+P310-P302+P352-P304+P340-P305+P351+P338-P308+P313-P311-P312-P330-P333+P313-P361-P363-P501c
View more
Phenylhydrazine hydrochloride is used as a selective mannosidase inhibitor. It is used in the study of tyrosine phosphorylation of janus protein tyrosine kinase in the EPO-responsive normal erythroblastoid cells of anemic mice. It is used in steroid assays and as an N-protecting reagent. Its free base, phenylhydrazine is used to prepare indoles, which find application as intermediates in the synthesis of various dyes and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenylhydrazine hydrochloride is used as a selective mannosidase inhibitor. It is used in the study of tyrosine phosphorylation of janus protein tyrosine kinase in the EPO-responsive normal erythroblastoid cells of anemic mice. It i
Phenylhydrazine hydrochloride is used as a selective mannosidase inhibitor. It is used in the study of tyrosine phosphorylation of janus protein tyrosine kinase in the EPO-responsive normal erythroblastoid cells of anemic mice. It i
General References:
- Butin, A. V.; Uchuskin, M. G.; Pilipenko, A. S.; Serdyuk, O. V.; Trushkov, I. V. Unusual reactivity of beta-(3-indolyl)-alpha, beta-unsaturated ketones. 2-Acetylvinyl group removal by phenylhydrazine hydrochloride. Tetrahedron Lett. 2011, 52 (41), 5255-5258.
- Muralirajan, K.; Haridharan, R.; Prakash, S.; Cheng, C. H. Rhodium(III)-Catalyzed in situ Oxidizing Directing Group-Assisted C H Bond Activation and Olefination: A Route to 2-Vinylanilines. Adv. Synth. Catal. 2015, 357, (4), 761-766.
