Search Thermo Fisher Scientific
Thermo Scientific Chemicals
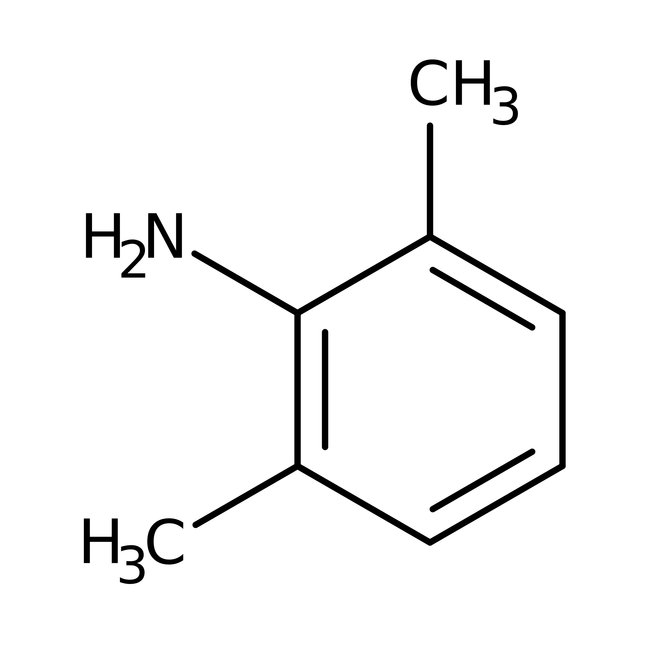
2,6-Dimethylaniline, 99%, Thermo Scientific Chemicals
CAS: 87-62-7 | C8H11N | 121.18 g/mol
Catalog number ALFA14512.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material2,6-Dimethylaniline
Melting Point11°C
CAS87-62-7
Health Hazard 1H227-H302+H312-H315-H319-H335-H351
Health Hazard 2GHS H Statement
H351-H302-H312-H332-H315-H335-H227
Suspected of causing cancer.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Causes skin irritation.
May cause respiratory irritation.
Combustible liquid.
H351-H302-H312-H332-H315-H335-H227
Suspected of causing cancer.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Causes skin irritation.
May cause respiratory irritation.
Combustible liquid.
View more
2,6-Dimethylaniline is used in pharmaceuticals, as dye intermediates and in organic synthesis. It is also used in the production of antioxidants, agricultural, pharmaceutical, rubber chemicals and other target organic molecules.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,6-Dimethylaniline is used in pharmaceuticals, as dye intermediates and in organic synthesis. It is also used in the production of antioxidants, agricultural, pharmaceutical, rubber chemicals and other target organic molecules.
Solubility
Slightly miscible with water. Miscible with ethanol and diethyl ether.
Notes
It is sensitive to air and light. Incompatible withacids, acid chlorides, acid anhydrides, oxidizing agents, chloroformates and halogens.
2,6-Dimethylaniline is used in pharmaceuticals, as dye intermediates and in organic synthesis. It is also used in the production of antioxidants, agricultural, pharmaceutical, rubber chemicals and other target organic molecules.
Solubility
Slightly miscible with water. Miscible with ethanol and diethyl ether.
Notes
It is sensitive to air and light. Incompatible withacids, acid chlorides, acid anhydrides, oxidizing agents, chloroformates and halogens.
RUO – Research Use Only
General References:
- Hindered aromatic amines do not form imines by direct reaction with benzophenones. An indirect route involves formation of the imine with an aldehyde, introduction of the second aryl group by 1,2-addition of the aryllithium and dehydrogenation of the resulting secondary amine with DDQ: J. Org. Chem., 54, 2464 (1989).
- Huang, W. C.; Lin, Y. C. ; Tzeng, W. B. Mass-analyzed threshold ionization spectroscopy of 2,6-dimethylaniline, 2,6-dimethylaniline-NHD, and 2,6-dimethylaniline-ND2. Chem. Phys. Lett. 2012, 551, 50-53.
- Ting, W-P.; Huang, Y-H.; Lu, M-C. Oxidation of 2,6-dimethylaniline by the Fenton, electro-Fenton and photoelectro-Fenton processes. J. Environ. Sci. Health, Part A 2011, 46 (10), 1085-1091.
- Gockel, S. N.; Hull, K. L. Chloroform as a Carbon Monoxide Precursor: In or Ex Situ Generation of CO for Pd-Catalyzed Aminocarbonylations. Org. Lett. 2015, 17 (13), 3236-3239.
- Yao, Q.; Wang, Z.; Zhang, Y.; Liu, X.; Lin, L.; Feng, X. N,N'-Dioxide/Gadolinium(III)-Catalyzed Asymmetric Conjugate Addition of Nitroalkanes to α, β-Unsaturated Pyrazolamides. J. Org. Chem. 2015, 80 (11), 5704-5712.