Search Thermo Fisher Scientific
Thermo Scientific Chemicals
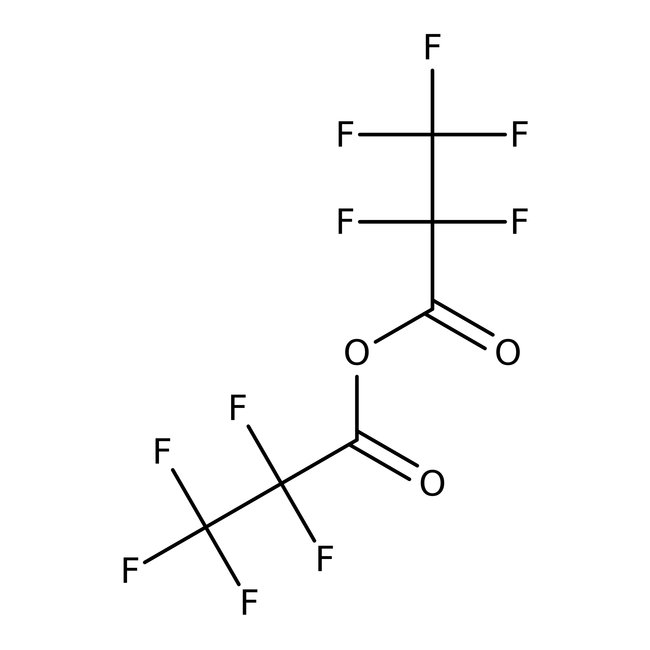
Pentafluoropropionic anhydride, 98%, Thermo Scientific Chemicals
CAS: 356-42-3 | C6F10O3 | 310.047 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14472.14 | 25 g |
Catalog number ALFA14472.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialPentafluoropropionic anhydride
CAS356-42-3
Health Hazard 1H314-H332-H335
Health Hazard 2GHS H Statement
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
H314-H318
Causes severe skin burns and eye damage.
Causes serious eye damage.
Health Hazard 3P260-P264b-P271-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
Pentafluoropropionic anhydride act as a derivatising agent of amines and alcohols. It is used in the in preparation of halogenated derivatives of phenolic acids, required for determination of some biologically important acids in cerebrospinal fluid, as derivatization reagent in detection of 1,2,3,4-tetrahydroisoquinoline and 1-methyl-1,2,3,4-tetrahydroisoquinoline as endogenous amines from non-treated rat brain by GC-multiple ion detection and in simultaneous determination of the major metabolites of dopamine in rat striatum by gas-liquid chromatography.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Pentafluoropropionic anhydride act as a derivatising agent of amines and alcohols. It is used in the in preparation of halogenated derivatives of phenolic acids, required for determination of some biologically important acids in cerebrospinal fluid, as derivatization reagent in detection of 1,2,3,4-tetrahydroisoquinoline and 1-methyl-1,2,3,4-tetrahydroisoquinoline as endogenous amines from non-treated rat brain by GC-multiple ion detection and in simultaneous determination of the major metabolites of dopamine in rat striatum by gas-liquid chromatography.
Solubility
Reacts with water.
Notes
Store at room temperature. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage.
Pentafluoropropionic anhydride act as a derivatising agent of amines and alcohols. It is used in the in preparation of halogenated derivatives of phenolic acids, required for determination of some biologically important acids in cerebrospinal fluid, as derivatization reagent in detection of 1,2,3,4-tetrahydroisoquinoline and 1-methyl-1,2,3,4-tetrahydroisoquinoline as endogenous amines from non-treated rat brain by GC-multiple ion detection and in simultaneous determination of the major metabolites of dopamine in rat striatum by gas-liquid chromatography.
Solubility
Reacts with water.
Notes
Store at room temperature. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage.
RUO – Research Use Only
General References:
- E Watson.; S Wilk. Derivatization and gas chromatographic determination of some biologically important acids in cerebrospinal fluid. Analytical Biochemistry. 1974, 59 (2), 441-451.
- M Kohno.; S Ohta.; M Hirobe. Tetrahydroisoquinoline and 1-methyl-tetrahydroisoquinoline as novel endogenous amines in rat brain. Biochemical and Biophysical Research Communications. 1986, 140 (1), 448-454.
- Reagent for the preparation of volatile pentafluoropropionyl derivatives of amines, alcohols, etc. for GC, sensitive to electron-capture detection: J. Chromat., 60, 134 (1971); 69, 157 (1972); 97, 19, 175 (1974); Anal. Chem., 56, 1695 (1984). For monographs on GC reagents, see: D. R. Knapp, Handbook of Analytical Derivatisation Reactions, Wiley, N.Y. (1979); Handbook of Derivatives for Chromatography, 2nd ed., K. Blau and J. M. Halket, Eds., Wiley, Chichester (1993).