Search Thermo Fisher Scientific
Thermo Scientific Chemicals
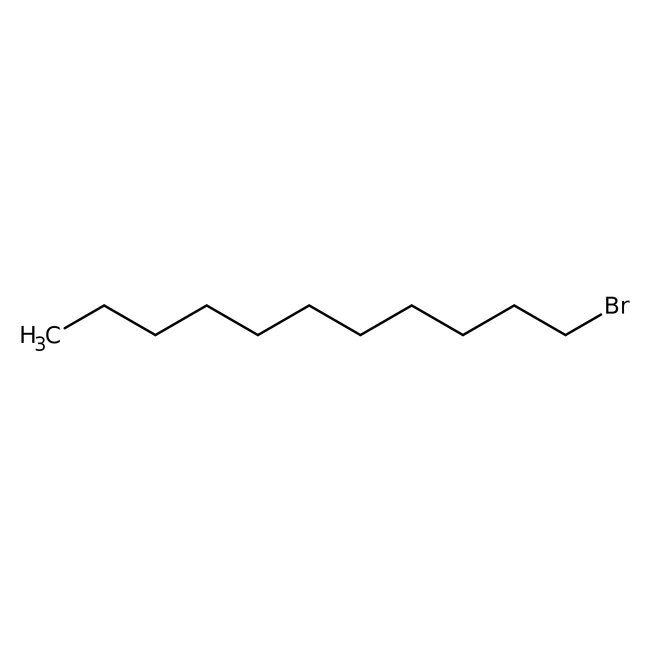
1-Bromoundecane, 98%, Thermo Scientific Chemicals
CAS: 693-67-4 | C11H23Br | 235.209 g/mol
Catalog number ALFA14333.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS7727-54-0
IUPAC Namediammonium [(sulfonatoperoxy)sulfonyl]oxidanide
Molecular FormulaH8N2O8S2
InChI KeyROOXNKNUYICQNP-UHFFFAOYSA-N
SMILES[NH4+].[NH4+].[O-]S(=O)(=O)OOS([O-])(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Appearance (Form)Crystalline powder or crystals
Titration with KMnO4>=98.0 %
Heavy metals (as Pb)=<0.005 %
Residue after ignition=<0.05 %
View more
1-Bromoundecane is used in the preparation of Grignard reagent by reacting with Mg in THF (tetrahydrofuran). It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromoundecane is used in the preparation of Grignard reagent by reacting with Mg in THF (tetrahydrofuran). It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong bases.
1-Bromoundecane is used in the preparation of Grignard reagent by reacting with Mg in THF (tetrahydrofuran). It is an important raw material and intermediate used in organic synthesis, pharmaceuticals, dyes and agrochemicals.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Kumaraswamy G.; Markondaiah B. Enantioselective total synthesis of (-)-tetrahydrolipstatin using Oppolzer’s sultam directed aldol reaction. Tetrahedron Lett. 2008, 49 (2), 327-330.
- Jean-François Peyrata.; Bruno Figadèrea.; André Cavé. Halide Exchange: Preparation of Alkyl Chlorides. Synthetic Communications: An International Journal for Rapid Communication of Synthetic Organic Chemistry. 1996, 26 (24), 4563-4567.