Search Thermo Fisher Scientific
Dimethyl methylphosphonate, 97%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA14268.22 | 100 g |
Catalog number ALFA14268.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
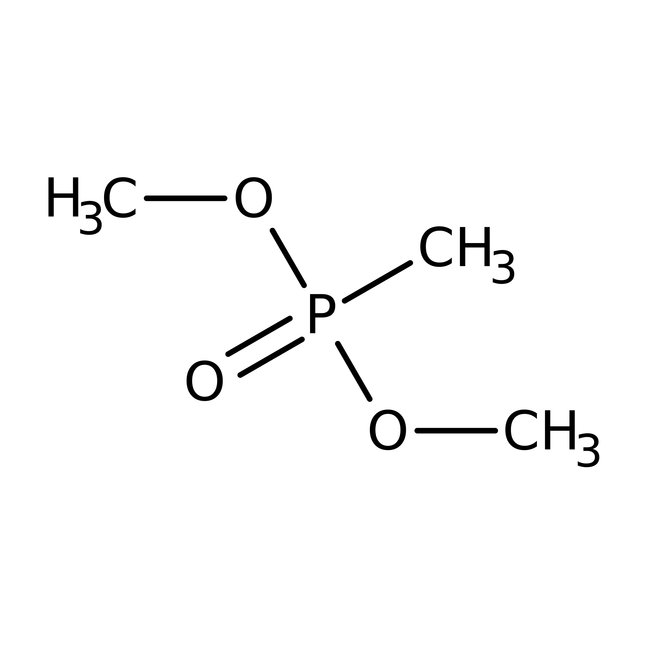
Chemical Name or MaterialDimethyl methylphosphonate
CAS756-79-6
Health Hazard 1H227-H319-H332-H335-H340-H360-H373
Health Hazard 2GHS H Statement
H340-H319-H227
May cause genetic defects.
Causes serious eye irritation.
Combustible liquid.
H340-H319-H227
May cause genetic defects.
Causes serious eye irritation.
Combustible liquid.
Health Hazard 3P201-P202-P210-P235-P260-P264b-P271-P280i-P281-P304+P340-P305+P351+P338-P308+P313-P370+P378q-P501c
View more
Dimethyl methylphosphonate is used as a catalyst and a reagent in organic synthesis for the conversion of esters to ketophosphonates. It is an additive for the synthesis of unsaturated polyester resin which has flame retardant high phosphorous and UV-cured epoxy acrylate. It finds application as hydraulic fluids as well.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dimethyl methylphosphonate is used as a catalyst and a reagent in organic synthesis for the conversion of esters to ketophosphonates. It is an additive for the synthesis of unsaturated polyester resin which has flame retardant high phosphorous and UV-cured epoxy acrylate. It finds application as hydraulic fluids as well.
Solubility
Miscible with water, ethanol, ether, alcohol, benzene, acetone and carbon tetrachloride. Immiscible with heavy mineral oil.
Notes
Incompatible materials strong oxidizing agents and strong bases.
Dimethyl methylphosphonate is used as a catalyst and a reagent in organic synthesis for the conversion of esters to ketophosphonates. It is an additive for the synthesis of unsaturated polyester resin which has flame retardant high phosphorous and UV-cured epoxy acrylate. It finds application as hydraulic fluids as well.
Solubility
Miscible with water, ethanol, ether, alcohol, benzene, acetone and carbon tetrachloride. Immiscible with heavy mineral oil.
Notes
Incompatible materials strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- The lithio-derivative reacts with esters to give ß-ketophosphonates, useful precursors of ɑß-unsaturated ketones by Horner-Wadsworth-Emmons olefination: J. Am. Chem. Soc., 88, 5654 (1966). An intramolecular version leads to cyclopentenones: Synth. Commun., 5, 1 (1975); Tetrahedron Lett., 22, 257 (1981). Dimethyl glutarate gives the 3-(phosphonatomethyl)cyclohexenone, olefination of which affords the corresponding 3-alkenylcycloalkenone. Dimethyl succinate also gives this reaction, but in lower yield; higher diesters give acyclic products: J. Org. Chem., 59, 1943 (1994):
- See also Diethyl methyl phosphonate, A14772 and Appendix 1.
- Park, E. J.; Han, S. W.; Jeong, B.; Park, S. H.; Kim, Y. G.; Kim, Y. H.; Kim, Y. D. Effect of polydimethylsiloxane (PDMS) coating on TiO2-based MALDI matrix for dimethyl methylphosphonate (DMMP) analysis. Appl. Surf. Sci. 2015, 353, 342-349.
- Yoo, R.; Cho, S.; Song, M. J.; Lee, W. Highly sensitive gas sensor based on Al-doped ZnO nanoparticles for detection of dimethyl methylphosphonate as a chemical warfare agent simulant. Sens. Actuators, B 2015, 221, 217-223.