Search Thermo Fisher Scientific
Thermo Scientific Chemicals
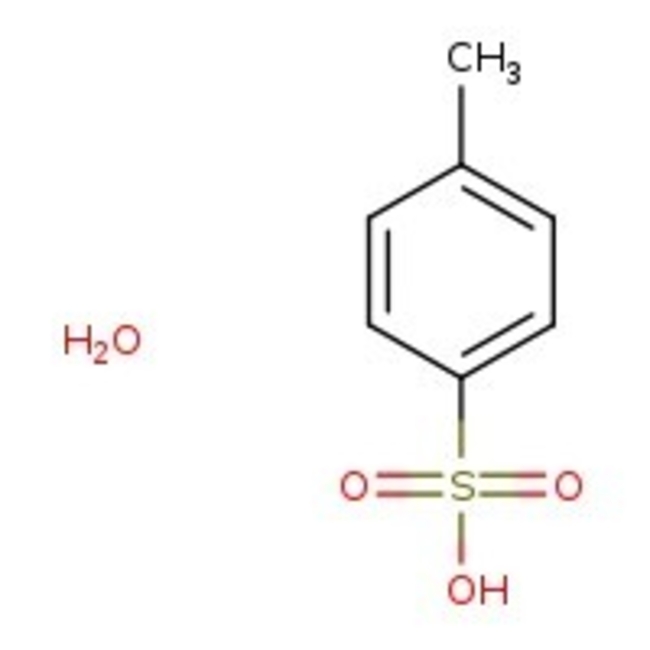
p-Toluenesulfonic acid monohydrate, 97%, Thermo Scientific Chemicals
CAS: 6192-52-5 | C7H10O4S | 190.21 g/mol
Catalog number ALFA14119.0B
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1000 g
Chemical Identifiers
CAS39394-84-8
IUPAC Namediphosphooxidane; methanesulfonic acid
Molecular FormulaCH4O8P2S
InChI KeyJHNLZOVBAQWGQU-UHFFFAOYSA-N
SMILESCS(O)(=O)=O.O=P(=O)OP(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Solution
Titration with NaOH6.5 to 10.0 % (P2O5)
Appearance (Color)Clear colorless to yellow to orange
AppearanceMay contain precipitate, re-dissolves on warming
Appearance (Color)to light brown or pale pink
View more
p-Toluenesulfonic acid monohydrate is used as a catalyst in the synthesis of resveratrol, in oxane derivatives as an antimalarial agent, as substituted piperidine and unsymmetrical benzyl. It acts as a catalyst in the preparation of 1,3,5-trisubstituted pyrazoles derivatives, selenated ketene dithioacetals, triazoloquinazolinone and benzimidazoquinazolinone derivatives. It also serves as an intermediate in the esterification and in reductive amination reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
p-Toluenesulfonic acid monohydrate is used as a catalyst in the synthesis of resveratrol, in oxane derivatives as an antimalarial agent, as substituted piperidine and unsymmetrical benzyl. It acts as a catalyst in the preparation of 1,3,5-trisubstituted pyrazoles derivatives, selenated ketene dithioacetals, triazoloquinazolinone and benzimidazoquinazolinone derivatives. It also serves as an intermediate in the esterification and in reductive amination reactions.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong bases.
p-Toluenesulfonic acid monohydrate is used as a catalyst in the synthesis of resveratrol, in oxane derivatives as an antimalarial agent, as substituted piperidine and unsymmetrical benzyl. It acts as a catalyst in the preparation of 1,3,5-trisubstituted pyrazoles derivatives, selenated ketene dithioacetals, triazoloquinazolinone and benzimidazoquinazolinone derivatives. It also serves as an intermediate in the esterification and in reductive amination reactions.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only