Search Thermo Fisher Scientific
Thermo Scientific Chemicals
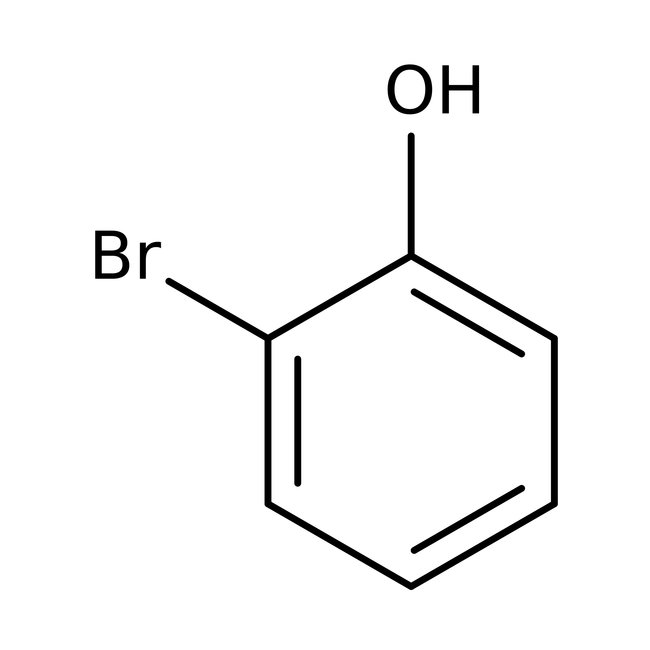
2-Bromophenol, 98%, Thermo Scientific Chemicals
CAS: 95-56-7 | C6H5BrO | 173.009 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14112.14 | 25 g |
Catalog number ALFA14112.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material2-Bromophenol
CAS95-56-7
Health Hazard 1H226-H302-H315-H319-H335
Health Hazard 2GHS H Statement
H302-H312-H315-H319-H335-H227
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H302-H312-H315-H319-H335-H227
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P270-P271-P280-P301+P312-P303+P361+P353-P304+P340-P305+P351+P338-P312-P330-P332+P313-P363-P370+P378q-P501c
View more
2-Bromophenol used as a disinfection byproduct found in chlorinated pool water. Used in the preparation of anti-benzofurobenzofuran diimides. It was also used to study the photodegradation of 2-bromophenol using UV-Vis spectroscopy and HPLC.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromophenol used as a disinfection byproduct found in chlorinated pool water. Used in the preparation of anti-benzofurobenzofuran diimides. It was also used to study the photodegradation of 2-bromophenol using UV-Vis spectroscopy and HPLC.
Solubility
Soluble in chloroform and ether. Slightly soluble in water.
Notes
Light sensitive. Store in dark. Store in cool, dry place in tightly closed containers.
2-Bromophenol used as a disinfection byproduct found in chlorinated pool water. Used in the preparation of anti-benzofurobenzofuran diimides. It was also used to study the photodegradation of 2-bromophenol using UV-Vis spectroscopy and HPLC.
Solubility
Soluble in chloroform and ether. Slightly soluble in water.
Notes
Light sensitive. Store in dark. Store in cool, dry place in tightly closed containers.
RUO – Research Use Only
General References:
- Sabin-Lucian Suraru et. al. Diindole-annulated naphthalene diimides: synthesis and optical and electronic properties of syn- and anti-isomers. Journal of Organic Chemistry. 2014, 79 (1), 128-139.
- Jayaraman A; Mas S; Tauler R, et al. Study of the photodegradation of 2-bromophenol under UV and sunlight by spectroscopic, chromatographic and chemometric techniques. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2012, 910 (1), 138-48.
- Reacts with n-BuLi to give a dilithio derivative, which reacts with electrophiles at carbon in a useful synthesis of ortho-substituted phenols: J. Org. Chem., 49, 5267 (1984).