Search Thermo Fisher Scientific
Thermo Scientific Chemicals
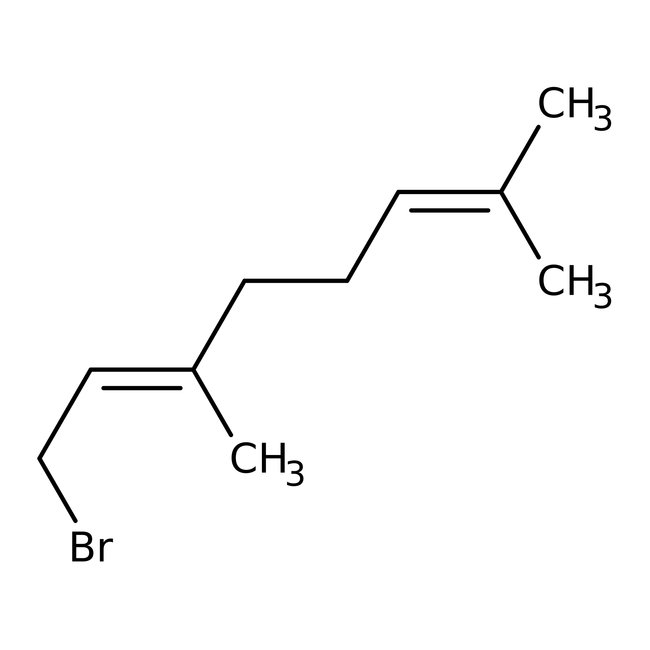
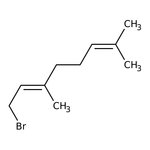
Geranyl bromide, 96%, Thermo Scientific Chemicals
CAS: 6138-90-5 | C10H17Br | 217.15 g/mol
Catalog number ALFA14093.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS617-86-7
IUPAC Nametriethylsilyl
Molecular FormulaC6H15Si
InChI KeyQXTIBZLKQPJVII-UHFFFAOYSA-N
SMILESCC[Si](CC)CC
View more
Specifications Specification Sheet
Specification Sheet
GC>=98.5 %
Refractive index1.4100 to 1.4120 (20°C, 589 nm)
Appearance (Color)Clear colorless
Appearance (Form)Liquid
Infrared spectrumConforms
Geranyl bromide is used for the preparation of 3,7-dihydroxyflavone derivatives and baicalein. It is also involved in the synthesis of potential flavonoidic modulators of P-glycoprotein activity. Further, it reacts with benzenesulfinic acid to prepare geranyl phenyl sulfone. In addition to this, it takes part in the palladium catalyzed cross coupling with aryl and alkenylgold(I) phosphanes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Geranyl bromide is used for the preparation of 3,7-dihydroxyflavone derivatives and baicalein. It is also involved in the synthesis of potential flavonoidic modulators of P-glycoprotein activity. Further, it reacts with benzenesulfinic acid to prepare geranyl phenyl sulfone. In addition to this, it takes part in the palladium catalyzed cross coupling with aryl and alkenylgold(I) phosphanes.
Solubility
Immiscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
Geranyl bromide is used for the preparation of 3,7-dihydroxyflavone derivatives and baicalein. It is also involved in the synthesis of potential flavonoidic modulators of P-glycoprotein activity. Further, it reacts with benzenesulfinic acid to prepare geranyl phenyl sulfone. In addition to this, it takes part in the palladium catalyzed cross coupling with aryl and alkenylgold(I) phosphanes.
Solubility
Immiscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Terpene building block.
- The Horner reaction with Methyl diphenyl phosphine oxide, A11484, provides a convenient access to the natural product (3Z,6E)-ɑ-farnesene, greatly superior to earlier multistep approaches: J. Org. Chem., 60, 6211 (1995):
- Schmidt, J.; Adrian, J.; Stark, C. B. W. Short and highly efficient synthesis of lipid peroxidation inhibitor pyrrolostatin and some analogues thereof. Org. Biomol. Chem. 2015, 13 (30), 8173-8176.
- Xu, L.; Liu, Z.; Dong, W.; Song, J.; Miao, M.; Xu, J.; Ren, H. Copper-free arylation of 3,3-disubstituted allylic halides with triazene-softened aryl Grignard reagents. Org. Biomol. Chem. 2015, 13 (22), 6333-6337.