Search Thermo Fisher Scientific
Thermo Scientific Chemicals
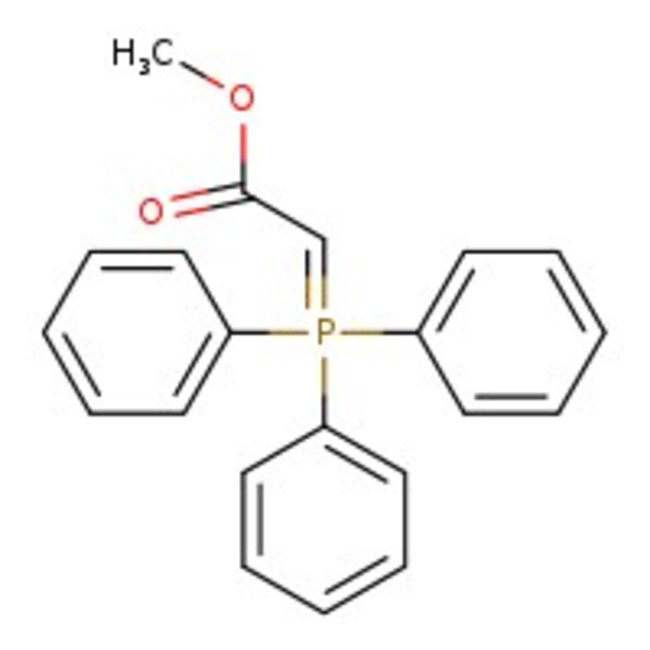
(Methoxycarbonylmethylene)triphenylphosphorane, 98%, Thermo Scientific Chemicals
CAS: 2605-67-6 | C21H19O2P | 334.36 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14020.14 | 25 g |
Catalog number ALFA14020.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material(Methoxycarbonylmethylene)triphenylphosphorane
CAS2605-67-6
Health Hazard 1H301-H315-H319-H335
Health Hazard 3P261-P264b-P270-P271-P280-P301+P310-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
Melting Point165°C to 169°C
View more
(Methoxycarbonylmethylene)triphenylphosphorane is used in olefination reactions. Further, it undergoes the Wittig reaction with aldehydes to give substituted methyl acrylates. It is used in the preparation of (triphenylphosphoranylidene)-ketene.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(Methoxycarbonylmethylene)triphenylphosphorane is used in olefination reactions. Further, it undergoes the Wittig reaction with aldehydes to give substituted methyl acrylates. It is used in the preparation of (triphenylphosphoranylidene)-ketene.
Solubility
Soluble in chloroform. Slightly soluble in ethanol and terahydrofuran. Insoluble in water.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
(Methoxycarbonylmethylene)triphenylphosphorane is used in olefination reactions. Further, it undergoes the Wittig reaction with aldehydes to give substituted methyl acrylates. It is used in the preparation of (triphenylphosphoranylidene)-ketene.
Solubility
Soluble in chloroform. Slightly soluble in ethanol and terahydrofuran. Insoluble in water.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Stable ylide which undergoes the Wittig reaction with aldehydes to give substituted methyl acrylates. See also (Ethoxycarbonyl methyl ene) triphenyl phosphorane, A12896 and Appendix 1. For a detailed study of the reaction with benzaldehyde, see J. Org. Chem., 59, 1126 (1994). For use of high pressure to increase the yield and trans-selectivity of reaction with aromatic aldehydes, see: Liebigs Ann. Chem., 2135 (1983).
- For tandem Wittig reaction and Cope rearrangement, see: J. Chem. Soc., Chem. Commun., 381 (1995):
- Strong base, for example Na bis(TMS)amide, brings about elimination of methanol, giving ketenylidenetriphenylphosphorane, which reacts with ɑ-hydroxy-ketones, e.g. in the steroid series, to give butenolides, by acylation and Wittig cyclization: Chem. Ber., 113, 2038 (1980):
- Review of phosphacumulene ylides: Angew. Chem. Int. Ed., 16, 349 (1977).
- Dierkes, G.; Bongartz, A.; Guth, H.; Hayen, H. Quality Evaluation of Olive Oil by Statistical Analysis of Multicomponent Stable Isotope Dilution Assay Data of Aroma Active Compounds. J. Agric. Food Chem., 2012, 60 (1), 394-401.
- Mears, P. R.; Thomas, E. J. Difluoroallylation using a 2-bromomethyl-1,1-difluoroalk-1-ene. Tetrahedron Lett. 2015, 56 (26), 3980-3981.