Search Thermo Fisher Scientific
Thermo Scientific Chemicals
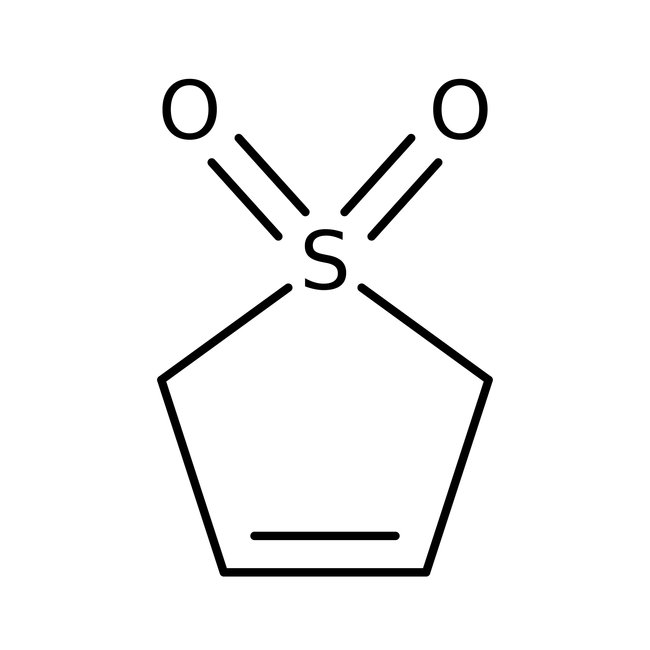
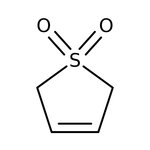
3-Sulfolene, 98%, Thermo Scientific Chemicals
CAS: 77-79-2 | C4H6O2S | 118.15 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13887.36 | 500 g |
Catalog number ALFA13887.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS120-14-9
IUPAC Name3,4-dimethoxybenzaldehyde
Molecular FormulaC9H10O3
InChI KeyWJUFSDZVCOTFON-UHFFFAOYSA-N
SMILESCOC1=CC=C(C=O)C=C1OC
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Melting point42°C to 45°C
GC>=99.0 %
Appearance (Color)White to yellow to light orange to tan
Appearance (Form)Powder or crystals or flakes and/or lumps
View more
It is a source of cisoid butadiene for Diels-Alder reactions. This chemical is also a convenient source of sulfur dioxide. It is a solvent used in the petrochemical industry for the extraction of aromatics from hydrocarbon streams.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is a source of cisoid butadiene for Diels-Alder reactions. This chemical is also a convenient source of sulfur dioxide. It is a solvent used in the petrochemical industry for the extraction of aromatics from hydrocarbon streams.
Solubility
Soluble in water, ethanol, benzene, ether.
Notes
Store in a cool, dry, well-ventilated area. Store away from oxidizing agent.
It is a source of cisoid butadiene for Diels-Alder reactions. This chemical is also a convenient source of sulfur dioxide. It is a solvent used in the petrochemical industry for the extraction of aromatics from hydrocarbon streams.
Solubility
Soluble in water, ethanol, benzene, ether.
Notes
Store in a cool, dry, well-ventilated area. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- Thomas E. SampleJr.; and Lewis F. Hatch. 3-Sulfolene: A butadiene source for a Diels-Alder synthesis: An undergraduate laboratory experiment. J. Chem. Educ. 1968, 45 (1), 55.
- Hans-Joachim Lehmler. Synthesis of environmentally relevant fluorinated surfactants—a review. Chemosphere. 2005, 58 (11), 1471-1496.
- Convenient crystalline source of 1,3-butadiene, which is generated on heating to about 110°C: Rec. Trav. Chim., 61, 785 (1942). For an example of its use in the Diels-Alder reaction, see: Org. Synth. Coll., 6, 454 (1988).
- Also behaves as a dienophile in with reactive dienes, for example 1,3-Diphenyl isobenzofuran, L00101: J. Org. Chem., 34, 538 (1969).
- In the presence of Pd(OAc)2, couples with aryldiazonium fluoroborates to give 3-aryl-4-sulfolenes which are readily isomerized with triethylamine to the 3-aryl-3-sulfolenes, providing a source of the corresponding 2-arylbutadienes: Synth. Commun., 26, 231 (1996).
- Lithiation occurs at the 2-position; subsequent alkylation and thermal extrusion of SO2 gave a polyene intermediate in a synthesis of the taxane ring system: J. Org. Chem., 62, 2957 (1997).