Search Thermo Fisher Scientific
Thermo Scientific Chemicals
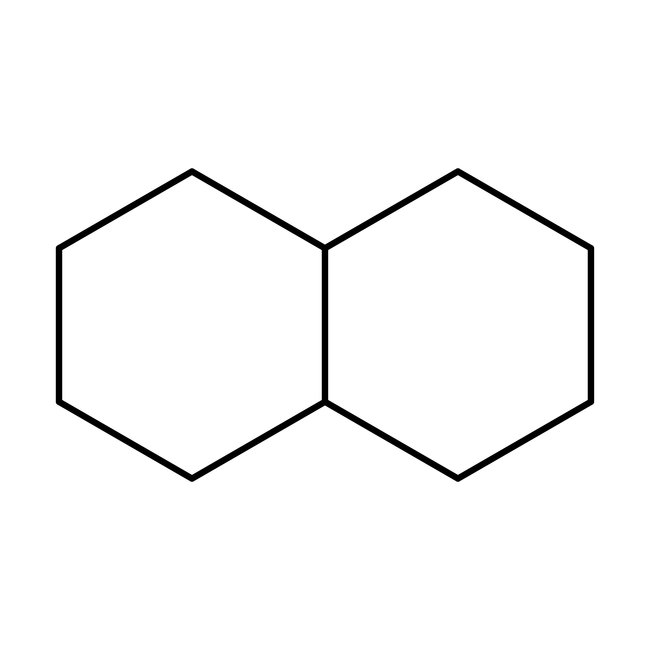
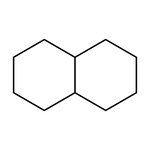
Decahydronaphthalene, cis + trans, 98%, Thermo Scientific Chemicals
CAS: 91-17-8 | C10H18 | 138.254 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13883.AP | 500 mL |
Catalog number ALFA13883.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Chemical Identifiers
CAS24470-78-8
IUPAC Nametriphenyl(propan-2-yl)phosphanium iodide
Molecular FormulaC21H22IP
InChI KeyHHBXWXJLQYJJBW-UHFFFAOYSA-M
SMILES[I-].CC(C)[P+](C1=CC=CC=C1)(C1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Melting point195°C to 200°C
Titration Argentometric97.5 to 102.5 %
Appearance (Form)Powder or crystalline powder or crystals
Appearance (Color)Light yellow to beige to light brown
Decahydronaphthalene is widely used as an industrial solvent for resins and fuel additives. It is a substitute for turpentine in lacquers, shoe polishes and waxes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Decahydronaphthalene is widely used as an industrial solvent for resins and fuel additives. It is a substitute for turpentine in lacquers, shoe polishes and waxes.
Solubility
Miscible with acetone, benzene, ether, methanol, aniline, decalin, chloroform, butanol and ethanol. Immiscible with water.
Notes
Store in cool place. Air, light and heat sensitive. Heat and light accelerate peroxide formation. Incompatible with oxidizing agents.
Decahydronaphthalene is widely used as an industrial solvent for resins and fuel additives. It is a substitute for turpentine in lacquers, shoe polishes and waxes.
Solubility
Miscible with acetone, benzene, ether, methanol, aniline, decalin, chloroform, butanol and ethanol. Immiscible with water.
Notes
Store in cool place. Air, light and heat sensitive. Heat and light accelerate peroxide formation. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Molina, A. I.; Gralberg, E.; Cecilia, J. A.; Finocchio, E.; Castellon, E. R. Nickel and cobalt phosphides as effective catalysts for oxygen removal of dibenzofuran: role of contact time, hydrogen pressure and hydrogen/feed molar ratio. Catal. Sci. Technol. 2015, 5 (6), 3403-3415.
- Lindemann, C.; Suchaux, P. D.; Naccoul, R. A.; Mokbel, I.; Malicet, V.; Jacques, J. Liquid-Liquid Equilibria at Three Temperatures (between 280.15 K and 333.15 K) of Binary, Ternary, and Quaternary Systems Involving Monoethylene Glycol, Water, Cyclohexane, para-Xylene, trans- and cis-Dimethylcyclohexane, and trans- and cis-Decahydronaphthalene. J. Chem. Eng. Data 2014, 59 (11), 3749-3755.