Search Thermo Fisher Scientific
Thermo Scientific Chemicals
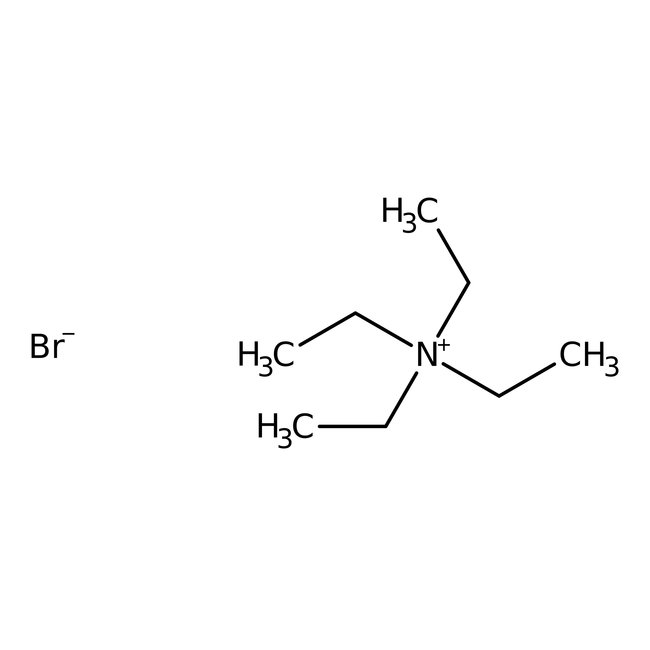
Tetraethylammonium bromide, 98%, Thermo Scientific Chemicals
CAS: 71-91-0 | C8H20BrN | 210.159 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13835.30 | 250 g |
Catalog number ALFA13835.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS34725-61-6
IUPAC Namesodium 3,5-dibromo-4-[3-(2,6-dibromo-4-hydroxyphenyl)-1,1-dioxo-3H-2,1λ⁶-benzoxathiol-3-yl]benzen-1-olate
Molecular FormulaC19H9Br4NaO5S
InChI KeyKGANHHDZDYZJEM-UHFFFAOYNA-M
SMILES[Na+].OC1=CC(Br)=C(C(Br)=C1)C1(OS(=O)(=O)C2=CC=CC=C12)C1=C(Br)C=C([O-])C=C1Br
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Clarity of solution(0.1% in water) clear
Appearance (Form)Powder
UV spectrumComplies
Visual transition intervalfrom pH 3.0 (yellow) to pH 4.6 (blue)
View more
Tetraethylammonium bromide is used as a phase transfer catalyst. It acts as a source of tetraethylammonium ions in pharmacological and physiological studies. It is also employed in organic chemical synthesis. It plays a major role in the preparation of tetraethylammonium superoxide from potassium superoxide for the conversion of primary alkyl halides to dialkyl peroxides. Furthermore, It is utilized as a catalyst in the oxidation of organic sulfide to sulfoxide using o-iodoxybenzoic acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetraethylammonium bromide is used as a phase transfer catalyst. It acts as a source of tetraethylammonium ions in pharmacological and physiological studies. It is also employed in organic chemical synthesis. It plays a major role in the preparation of tetraethylammonium superoxide from potassium superoxide for the conversion of primary alkyl halides to dialkyl peroxides. Furthermore, It is utilized as a catalyst in the oxidation of organic sulfide to sulfoxide using o-iodoxybenzoic acid.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and bases.
Tetraethylammonium bromide is used as a phase transfer catalyst. It acts as a source of tetraethylammonium ions in pharmacological and physiological studies. It is also employed in organic chemical synthesis. It plays a major role in the preparation of tetraethylammonium superoxide from potassium superoxide for the conversion of primary alkyl halides to dialkyl peroxides. Furthermore, It is utilized as a catalyst in the oxidation of organic sulfide to sulfoxide using o-iodoxybenzoic acid.
Solubility
Soluble in water.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and bases.
RUO – Research Use Only
General References:
- Phase-transfer catalyst; see Appendix 2.
- For use in combination with anhydrous HBr as a reagent for selective monohydrobromination of an alkyne, see: Org. Synth., 76, 263 (1998).
- Takemoto, K.; Yamada, H. Development of rechargeable lithium-bromine batteries with lithium ion conducting solid electrolyte. J. Power Sources 2015, 281, 334-340.
- Abdel-Rahman, L. H.; El-Khatib, R. M.; Nassr, L. A.; Abu-Dief, A. M. Salts and Structural Effects on the base Catalyzed Hydrolysis of some Novel and Pharmacologically Active Iron(II) Azomethine Amino Acid Complexes. Int. J. Nano. Chem. 2015, 1 (1), 25-30.