Search Thermo Fisher Scientific
Thermo Scientific Chemicals
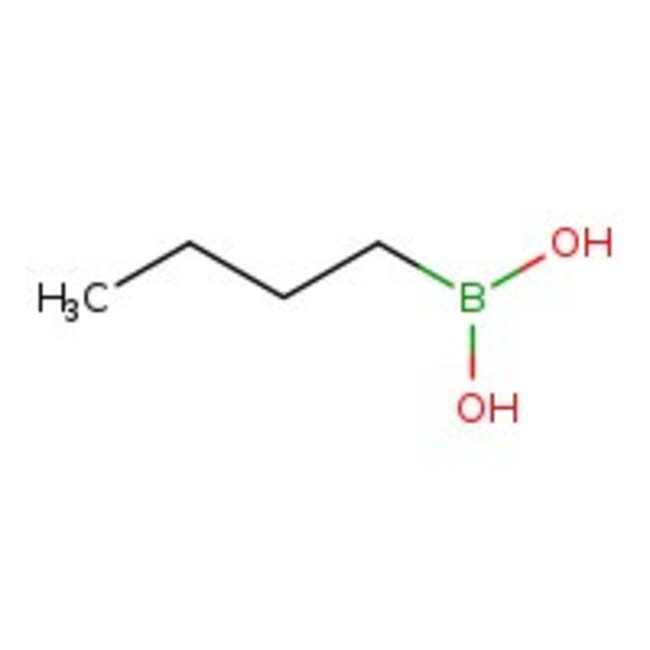
1-Butylboronic acid, 98%, Thermo Scientific Chemicals
CAS: 4426-47-5 | C4H11BO2 | 101.94 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13725.06 | 5 g |
Catalog number ALFA13725.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material1-Butylboronic acid
CAS4426-47-5
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
1-Butylboronic acid is used as a reagent for preparation of volatile derivatives of diols, e.g. carbohydrates, for GC and mass spectrometry and to prepare chiral oxazaborolidines. It is a precursor to unsymmetric borinic acids, which are inhibitors of serine proteases. Can be used as a transport carrier in membrane-based sugar separations and as an analytical reagent in the determination of serum glucose.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Butylboronic acid is used as a reagent for preparation of volatile derivatives of diols, e.g. carbohydrates, for GC and mass spectrometry and to prepare chiral oxazaborolidines. It is a precursor to unsymmetric borinic acids, which are inhibitors of serine proteases. Can be used as a transport carrier in membrane-based sugar separations and as an analytical reagent in the determination of serum glucose.
Solubility
Soluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. It is hygroscopic in nature. Incompatible with oxidizing agents. Store under dry inert gas.
1-Butylboronic acid is used as a reagent for preparation of volatile derivatives of diols, e.g. carbohydrates, for GC and mass spectrometry and to prepare chiral oxazaborolidines. It is a precursor to unsymmetric borinic acids, which are inhibitors of serine proteases. Can be used as a transport carrier in membrane-based sugar separations and as an analytical reagent in the determination of serum glucose.
Solubility
Soluble in water.
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. It is hygroscopic in nature. Incompatible with oxidizing agents. Store under dry inert gas.
RUO – Research Use Only
General References:
- Franck Picard.; Tobias Schulz.; Rolf W. Hartmann. 5-Phenyl substituted 1-methyl-2-pyridones and 4'-substituted biphenyl-4-carboxylic acids. synthesis and evaluation as inhibitors of steroid-5α-reductase type 1 and 2. Bioorganic & Medicinal Chemistry. 2002, 10 (2), 437-448.
- Zhao Yongliang.; Zhao Fengying.; Li Qiang.; Gao Deqing. Synthesis, Characterization and Fluorescence Properties of Europium, Terbium Complexes with Biphenyl-4-Carboxylic Acid and o-Phenanthroline. Journal of Rare Earths. 2006, 24 (1), 18-22.
- Reagent for preparation of volatile derivatives of diols, e.g. carbohydrates, for GC and mass spectrometry: J. Chromat., 54, 193 (1971); J. Chromat. Sci., 9, 18 (1971); Gas Chromat., 129 (1968).
- In combination with (S)-(-)-ɑ,ɑ-Diphenyl prolinol, L09217, or its enantiomer (9218), oxazaborolidine catalysts are generated in situ, for use in highly enantioselective reductions: Tetrahedron Lett., 33, 4141 (1992). The same system has been employed in the synthesis of chiral monosubstituted oxiranes: Tetrahedron Lett., 34, 5227 (1993). See also (S)-2-Methyl-CBS-oxazaborolidine monohydrate, L09219, and Appendix 5.