Search Thermo Fisher Scientific
Thermo Scientific Chemicals
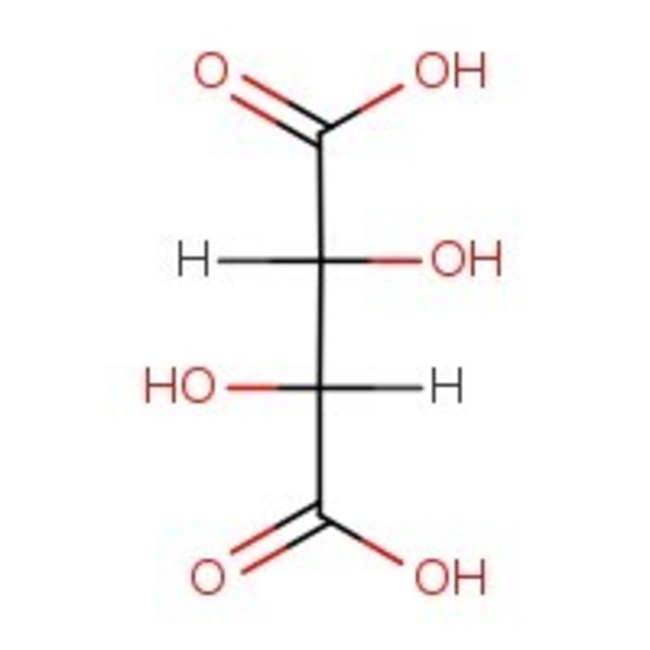
L-(+)-Tartaric acid, 99%, Thermo Scientific Chemicals
CAS: 87-69-4 | C4H6O6 | 150.09 g/mol
Catalog number ALFA13668.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS20605-01-0
IUPAC Name1,3-diethyl 2,2-bis(hydroxymethyl)propanedioate
Molecular FormulaC9H16O6
InChI KeyWIOHBOKEUIHYIC-UHFFFAOYSA-N
SMILESCCOC(=O)C(CO)(CO)C(=O)OCC
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder
Identification (FTIR)Conforms
Appearance (Color)White to pale cream
Assay (GC)≥94.0%
Melting Point (clear melt)45.0-55.0°C
L-(+)-Tartaric acid is widely utilized in pharmaceutical industries. It is used in soft drinks, confectionaries, food products, gelatin desserts and as a buffering agent. It forms a compound, TiCl2(O-i-Pr)2 with Diels-Alder catalyst and acta as a chelate agent in metal industries. Owing to its efficient chelating property towards metal ions, it is used in farming and metal industries for complexing micronutrients and for cleaning metal surfaces, respectively.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
L-(+)-Tartaric acid is widely utilized in pharmaceutical industries. It is used in soft drinks, confectionaries, food products, gelatin desserts and as a buffering agent. It forms a compound, TiCl2(O-i-Pr)2 with Diels-Alder catalyst and acta as a chelate agent in metal industries. Owing to its efficient chelating property towards metal ions, it is used in farming and metal industries for complexing micronutrients and for cleaning metal surfaces, respectively.
Solubility
Soluble in water, methanol and acetone.
Notes
Incompatible with oxidizing agents, bases and reducing agents.
L-(+)-Tartaric acid is widely utilized in pharmaceutical industries. It is used in soft drinks, confectionaries, food products, gelatin desserts and as a buffering agent. It forms a compound, TiCl2(O-i-Pr)2 with Diels-Alder catalyst and acta as a chelate agent in metal industries. Owing to its efficient chelating property towards metal ions, it is used in farming and metal industries for complexing micronutrients and for cleaning metal surfaces, respectively.
Solubility
Soluble in water, methanol and acetone.
Notes
Incompatible with oxidizing agents, bases and reducing agents.
RUO – Research Use Only
General References:
- Resolving agent for chiral bases. For improved resolution technique using two immiscible solvents and half equivalent of resolving agent, claimed to give higher optical purities and faster resolutions than conventional methods, see: Tetrahedron, 41, 2465 (1985).
- In combination with NaBH 4, induces asymmetric reduction of functionalized ketones with ee figures approaching those achieved with more expensive reagents: J. Chem. Soc. Perkin 1, 1826 (1990).
- Preferred acid catalyst, in combination with MgSO 4, for acetalization of unsaturated aldehydes with ethylene glycol, giving less double bond isomerization than stronger acids including tosic and succinic: J. Org. Chem., 60, 2931 (1995).
- Mostowicz, D.; Dygas, M.; Kałuża, Z. Heck Cyclization Strategy for Preparation of Erythrinan Alkaloids: Asymmetric Synthesis of Unnatural (-)-Erysotramidine from L-Tartaric Acid. J. Org. Chem. 2015, 80 (3), 1957-1963.
- Song, G.; Xu, C.; Li, B. Visual chiral recognition of mandelic acid enantiomers with l-tartaric acid-capped gold nanoparticles as colorimetric probes. Sens. Actuators B 2015, 215, 504-509.