Search Thermo Fisher Scientific
Thermo Scientific Chemicals
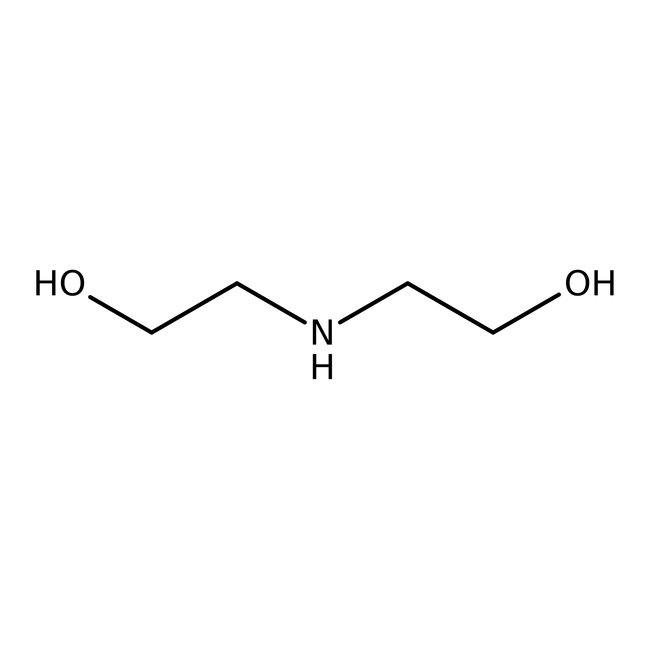
Diethanolamine, 99%, Thermo Scientific Chemicals
CAS: 111-42-2 | C4H11NO2 | 105.14 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13389.30 | 250 g |
Catalog number ALFA13389.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS4549-32-0
IUPAC Name1,8-dibromooctane
Molecular FormulaC8H16Br2
InChI KeyDKEGCUDAFWNSSO-UHFFFAOYSA-N
SMILESBrCCCCCCCCBr
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥97.5%
Refractive Index1.4975-1.5015 @ 20?C
FormLiquid
Identification (FTIR)Conforms
Diethanolamine is used in the preparation of morpholine and diethanolamides, which is an active ingredient in cosmetics and shampoos. It acts as a surfactant and a corrosion inhibitor. It is utilized to remove hydrogen sulfide and carbon dioxide from natural gas. Further, it is an intermediate used in the rubber chemicals, as a humectant and a softening agent, and as an emulsifier and dispersing agent in agricultural chemicals. In addition to this, it is used in cutting oils, cleaners, soaps, polishers, and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethanolamine is used in the preparation of morpholine and diethanolamides, which is an active ingredient in cosmetics and shampoos. It acts as a surfactant and a corrosion inhibitor. It is utilized to remove hydrogen sulfide and carbon dioxide from natural gas. Further, it is an intermediate used in the rubber chemicals, as a humectant and a softening agent, and as an emulsifier and dispersing agent in agricultural chemicals. In addition to this, it is used in cutting oils, cleaners, soaps, polishers, and pharmaceuticals.
Solubility
Miscible with water.
Notes
Hygroscopic and air sensitive. Incompatible with strong oxidizing agents, strong acids, copper, zinc, carbon dioxide and iron.
Diethanolamine is used in the preparation of morpholine and diethanolamides, which is an active ingredient in cosmetics and shampoos. It acts as a surfactant and a corrosion inhibitor. It is utilized to remove hydrogen sulfide and carbon dioxide from natural gas. Further, it is an intermediate used in the rubber chemicals, as a humectant and a softening agent, and as an emulsifier and dispersing agent in agricultural chemicals. In addition to this, it is used in cutting oils, cleaners, soaps, polishers, and pharmaceuticals.
Solubility
Miscible with water.
Notes
Hygroscopic and air sensitive. Incompatible with strong oxidizing agents, strong acids, copper, zinc, carbon dioxide and iron.
RUO – Research Use Only
General References:
- Boronic acids can be protected as cyclic boronates which are generally high-melting crystalline solids: J. Am. Chem. Soc., 77, 2491 (1955). NMR evidence has been presented indicating that derivatives of this type have a transannular B-N bond: J. Organomet. Chem., 246, 213 (1983):
- For use in the modification of a boronic acid cleft in formation of a sodium-saccharide cotransporter, see: J. Org. Chem., 60, 2147 (1995). For use of the butyl cyclic boronate in the formation of a chiral dioxaborolane ligand, see: Org. Synth., 76, 86 (1998). See also Appendix 6.
- Hickman, D. A.; Mosner, K.; Ringer, J. W. A continuous diethanolamine dehydrogenation fixed bed catalyst and reactor system. Chem. Eng. J. 2015, 278, 447-453.
- Bezgin, F.; Ayaz, N.; Demirelli, K. Synthesis, characterization, and dielectric properties of polymers functionalized with coumarone and diethanolamine. J. Appl. Polym. Sci. 2015, 132 (26), 42164.
- Davarpanah, M.; Ahmadpour, A.; Rohani-Bastami, T.; Dabir, H. Synthesis and application of diethanolamine-functionalized polystyrene as a new sorbent for the removal of p-toluenesulfonic acid from aqueous solution. J. Ind. Eng. Chem. 2015, 30, 281-288.