Search Thermo Fisher Scientific
Thermo Scientific Chemicals
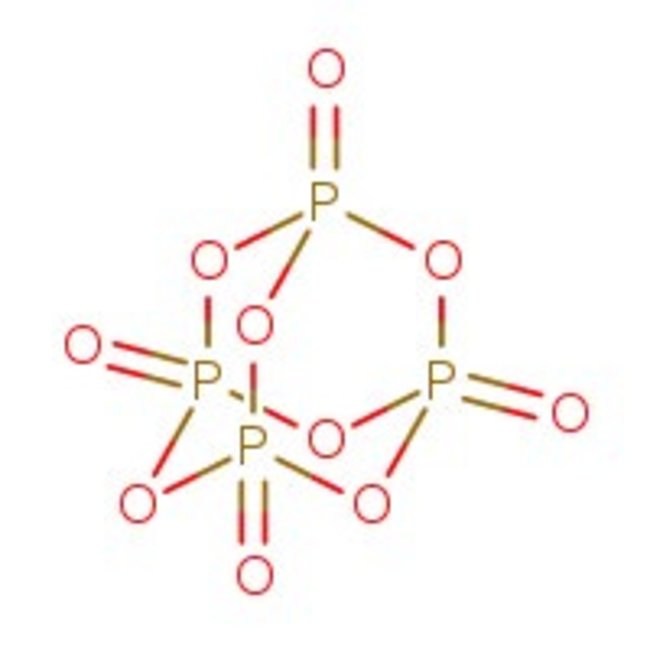
Phosphorus(V) oxide, 98%, Thermo Scientific Chemicals
CAS: 1314-56-3 | O5P2 | 141.94 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13348.36 | 500 g |
Catalog number ALFA13348.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS112275-50-0
IUPAC Nametert-butyl 1,4-diazepane-1-carboxylate
Molecular FormulaC10H20N2O2
InChI KeyWDPWEXWMQDRXAL-UHFFFAOYSA-N
SMILESCC(C)(C)OC(=O)N1CCCNCC1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
Assay (unspecified)≥97.5%
Refractive Index1.4680-1.4720 @ 20°C (non-U.S. specification)
FormLiquid
CommentSpecification differs for U.S. and non-U.S. material where indicated
Phosphorus(V) oxide is used as a drying and dehydrating agent, a condensation reagent in organic synthesis and a laboratory reagent. It is also used in sugar refining and in fire extinguishing. Phosphorus pentoxide in DMSO forms an Onodera reagent which oxidizes alcohols. It is capable of converting mineral acids to anhydrides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phosphorus(V) oxide is used as a drying and dehydrating agent, a condensation reagent in organic synthesis and a laboratory reagent. It is also used in sugar refining and in fire extinguishing. Phosphorus pentoxide in DMSO forms an Onodera reagent which oxidizes alcohols. It is capable of converting mineral acids to anhydrides.
Solubility
Soluble in sulfuric acid. Insoluble in acetone and ammonia. Decomposes in water.
Notes
It is highly hygroscopic in nature and gets hydrolyzed to phosphoric acid. It is stored in a separate safety cabinet away from incompatibles such as alkalis and water.
Phosphorus(V) oxide is used as a drying and dehydrating agent, a condensation reagent in organic synthesis and a laboratory reagent. It is also used in sugar refining and in fire extinguishing. Phosphorus pentoxide in DMSO forms an Onodera reagent which oxidizes alcohols. It is capable of converting mineral acids to anhydrides.
Solubility
Soluble in sulfuric acid. Insoluble in acetone and ammonia. Decomposes in water.
Notes
It is highly hygroscopic in nature and gets hydrolyzed to phosphoric acid. It is stored in a separate safety cabinet away from incompatibles such as alkalis and water.
RUO – Research Use Only
General References:
- Silylates primary and sec-alcohols and phenols, in the presence of p-TsOH as catalyst. If the substrate contains acid-sensitive groups, PPTS can be used: Tetrahedron Lett., 4261 (1978). See Appendix 4.
- In the presence of FeCl3, reacts with benzotrichlorides to give substituted benzoyl chlorides in good yields: J. Chem. Soc., Chem. Commun., 808 (1977).
- Reacts with P2O5 to give trimethylsilyl polyphosphate (PPSE), a milder and more convenient dehydrating agent then polyphosphoric acid. For examples, and related reagents, see Phosphorus(V) oxide, A13348.
- Meier, M. S. Phosphorus(V) Oxide. In Encyclopedia of Reagents for Organic Synthesis; Pacquette, L., Ed.; J. Wiley & Sons, New York, 2004.
- Ebadi, M.; Mirdamadian, Z.; Ghanbari, D.; Moradi, L. The Effect of Aminated Carbon Nanotube and Phosphorus Pentoxide on the Thermal Stability and Flame Retardant Properties of the Acrylonitrile-Butadiene-Styrene. J. Cluster Sci. 2014, 25 (2), 541-548.