Search Thermo Fisher Scientific
Thermo Scientific Chemicals
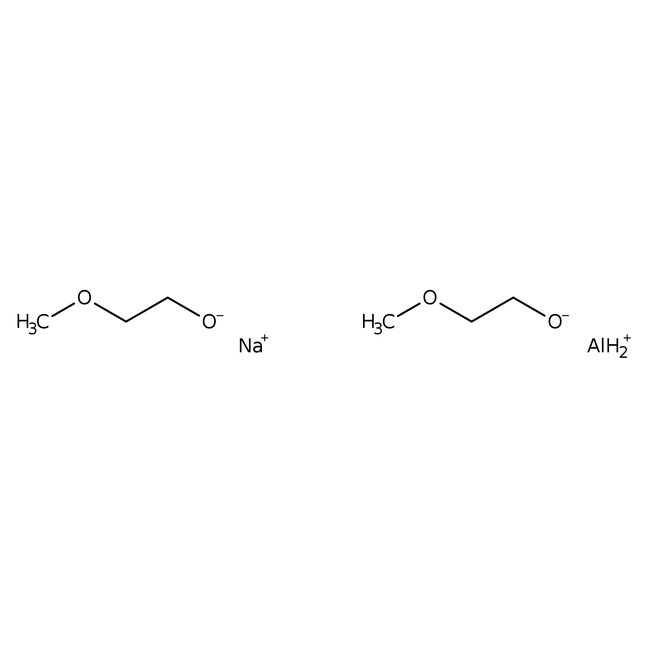
Sodium bis(2-methoxyethoxy)aluminum hydride, 70% w/w in toluene., Thermo Scientific Chemicals
CAS: 22722-98-1 | C6H16AlNaO4
| Catalog Number | Quantity |
|---|---|
| ALFA13292.30 | 250 g |
Catalog number ALFA13292.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialSodium bis(2-methoxyethoxy)aluminum hydride
CAS22722-98-1
Health Hazard 1H225-H304-H314-H318-H361-H373-X
Health Hazard 2GHS H Statement
H225-H260-H304-H361-H373-H314-H332-H336
Highly flammable liquid and vapour.
In contact with water releases flammable gases which may ignite spontaneously.
May be fatal if swallowed and enters airways.
Suspected of damaging fertility or the unborn child.
May cause damage to organs through prolonged or repeated exposure.
Causes severe skin burns and eye damage.
Harmful if inhaled.
May cause drowsiness or dizziness.
H225-H260-H304-H361-H373-H314-H332-H336
Highly flammable liquid and vapour.
In contact with water releases flammable gases which may ignite spontaneously.
May be fatal if swallowed and enters airways.
Suspected of damaging fertility or the unborn child.
May cause damage to organs through prolonged or repeated exposure.
Causes severe skin burns and eye damage.
Harmful if inhaled.
May cause drowsiness or dizziness.
Health Hazard 3P201-P202-P210-P233-P240-P241-P242-P243-P261-P262-P264b-P271-P280-P301+P310-P303+P361+P353-P304+P340-P305+P351+P338-P310-P312-P330-P331-P363-P372-P374-P380-P501c
View more
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
Solubility
Miscible with aromatic hydrocarbons, ether, tetrahydrofuran, dimethyl ether and dimethylformamide. Immiscible with aliphatic hydrocarbons.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with water, oxidizing agents and combustible material.
Sodium bis(2-methoxyethoxy)aluminum hydride acts as a reducing agent in organic synthesis. It is used to prepare azoxyarenes, azoarenes and hydroazoarenes from nitroarenes. It plays an important role for the conversion of aldehydes, ketones, carboxylic acids, esters, acyl halides and anhydrides to primary alcohols. It is also employed in hydroaluminate alkenes and alkynes. It is utilized in the reduction of lactones and epoxides into diols. Further, it serves as a methylation reagent for aryl-activated compounds. In addition to this, it is involved in the reduction of amides, nitriles and amines to the corresponding amines.
Solubility
Miscible with aromatic hydrocarbons, ether, tetrahydrofuran, dimethyl ether and dimethylformamide. Immiscible with aliphatic hydrocarbons.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with water, oxidizing agents and combustible material.
RUO – Research Use Only
General References:
- Convenient alternative to lithium aluminum hydride for many reductions: Coll. Czech. Chem. Commun., 34, 118 (1969). The reagent is more readily and safely handled, is more stable to air and can be used at high temperatures. For use in the stereospecific reduction of 2-yn-1-ols to (E)-allylic alcohols, and reviews, see: Org. Synth. Coll., 7, 524 (1990):
- The reducing properties can be modified by addition of one mole of ethanol, which gives a reagent for the reduction of lactones to lactols, or one mole of N-methylpiperazine or morpholine, which gives a reagent for the high-yield reduction of esters to aldehydes: Synthesis, 526 (1976).
- For use as a superior activator/initiator for the formation of Grignard reagents, see: Coll. Czech. Chem. Commun., 38, 1614 (1973).
- Xu, J. B.; Cheng, K. J. Studies on the Alkaloids of the Calycanthaceae and Their Syntheses. Molecules 2015, 20 (4), 6715-6738.
- Hatano, M.; Yamashita, K.; Mizuno, M.; Ito, O.; Ishihara, K. C-Selective and Diastereoselective Alkyl Addition to beta, gamma-Alkynyl-alpha-imino Esters with Zinc(II)ate Complexes. Angew. Chem. Int. Ed. 2015, 127 (9), 2745-2749.