Search Thermo Fisher Scientific
Thermo Scientific Chemicals
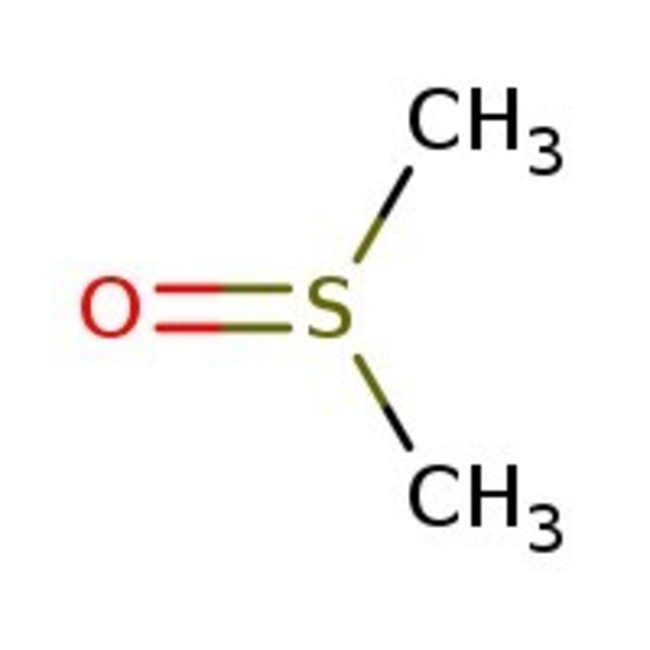
Dimethyl sulfoxide, 99+%, Thermo Scientific Chemicals
CAS: 67-68-5 | C2H6OS | 78.13 g/mol
Catalog number ALFA13280.0E
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 g
Chemical Identifiers
CAS615-74-7
IUPAC Name2-chloro-5-methylphenol
Molecular FormulaC7H7ClO
InChI KeySMFHPCZZAAMJJO-UHFFFAOYSA-N
SMILESCC1=CC=C(Cl)C(O)=C1
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder or fused solid or chunks
Assay (GC)≥98.5%
Appearance (Color)White to cream to yellow to pale brown
Melting Point (clear melt)43.0-49.0?C
Dimethyl Sulfoxide (DMSO) is used as a solvent for chemical reactions involving salts, most notably in Finkelstein reactions and in other nucleophilic substitutions. It is also used in antifreeze or hydraulic fluids and utilized in the oxidation of thiols and disulfides to sulfonic acids.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dimethyl Sulfoxide (DMSO) is used as a solvent for chemical reactions involving salts, most notably in Finkelstein reactions and in other nucleophilic substitutions. It is also used in antifreeze or hydraulic fluids and utilized in the oxidation of thiols and disulfides to sulfonic acids. It is also used as a cryoprotectant and is added to cell media to reduce ice formation and thereby prevent cell death during the freezing process.
Solubility
Miscible with water, ethanol, ether, benzene, chloroform and acetone.
Notes
Hygroscopic. Incompatible with acid chlorides, phosphorus halides, strong acids, strong oxidizing agents and strong reducing agents.
Dimethyl Sulfoxide (DMSO) is used as a solvent for chemical reactions involving salts, most notably in Finkelstein reactions and in other nucleophilic substitutions. It is also used in antifreeze or hydraulic fluids and utilized in the oxidation of thiols and disulfides to sulfonic acids. It is also used as a cryoprotectant and is added to cell media to reduce ice formation and thereby prevent cell death during the freezing process.
Solubility
Miscible with water, ethanol, ether, benzene, chloroform and acetone.
Notes
Hygroscopic. Incompatible with acid chlorides, phosphorus halides, strong acids, strong oxidizing agents and strong reducing agents.
RUO – Research Use Only
General References:
- Dipolar aprotic solvent with advantages over e.g. N,N-Dimethylformamide, A13547, 1-Methyl-2-pyrrolidinone, A12260 of lower toxicity and generally higher solvent power for many types of material.
- Powerful solvent for many inorganic ions, primarily due to solvation of the cations with consequent enhanced reactivity of the counter anions both in increased nucleophilicity and base strength. In DMSO, the relative nucleophilicities of the halide ions are reversed (F- > I-). The rate of aromatic fluorodenitration in a range of polar aprotic solvents was highest for DMSO: J. Fluorine Chem., 35, 591 (1987); see Potassium fluoride, 14130. For enhanced base strength, see e.g.: cyclization of ω-bromo acids to lactones by K2CO3: Org. Synth. Coll., 6, 698 (1988); exhaustive alkylation of ketones with KOH: Tetrahedron Lett., 31, 859 (1990).