Search Thermo Fisher Scientific
Thermo Scientific Chemicals
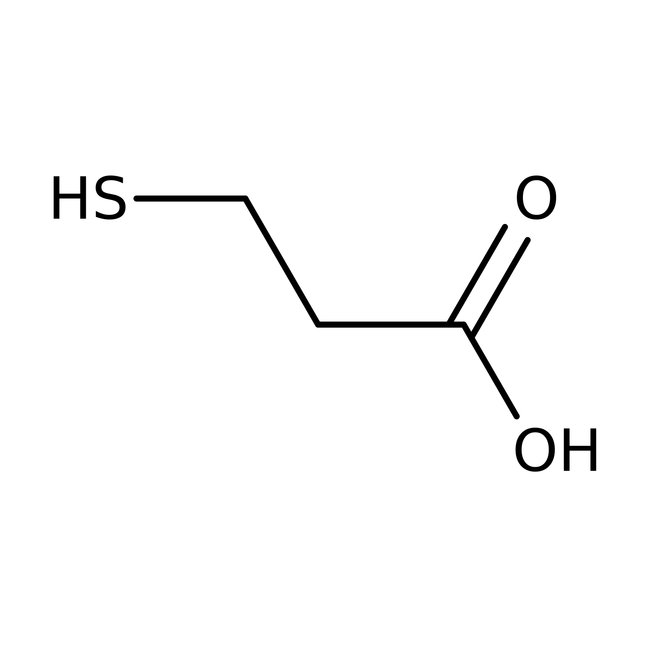
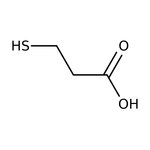
3-Mercaptopropionic acid, 99%, Thermo Scientific Chemicals
CAS: 107-96-0 | C3H6O2S | 106.139 g/mol
Catalog number ALFA13261.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS21055-37-8
IUPAC Namemethyl 2-isothiocyanatoacetate
Molecular FormulaC4H5NO2S
InChI KeyGOWGDPFDGIPFIK-UHFFFAOYSA-N
SMILESCOC(=O)CN=C=S
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Yellow to brown
Assay (GC)≥97.5%
Refractive Index1.5170-1.5210 @ 20°C
FormLiquid
3-Mercaptopropionic acid is widely used in food and beverage industries as a flavoring agent. It is used in the production of PVC stabilizers, which are used as chain transfer agents in polymerizations. It can be used as primary or secondary, color stabilizer in combination with phenolic antioxidant for polymers. It acts as a sulfide ion equivalent and is utilized in the preparation of diaryl sulfide from aryl iodide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Mercaptopropionic acid is widely used in food and beverage industries as a flavoring agent. It is used in the production of PVC stabilizers, which are used as chain transfer agents in polymerizations. It can be used as primary or secondary, color stabilizer in combination with phenolic antioxidant for polymers. It acts as a sulfide ion equivalent and is utilized in the preparation of diaryl sulfide from aryl iodide.
Solubility
Miscible with water, alcohol, benzene and ether.
Notes
Air Sensitive. Hygroscopic. Incompatible with bases, oxidizing agents and reducing agents.
3-Mercaptopropionic acid is widely used in food and beverage industries as a flavoring agent. It is used in the production of PVC stabilizers, which are used as chain transfer agents in polymerizations. It can be used as primary or secondary, color stabilizer in combination with phenolic antioxidant for polymers. It acts as a sulfide ion equivalent and is utilized in the preparation of diaryl sulfide from aryl iodide.
Solubility
Miscible with water, alcohol, benzene and ether.
Notes
Air Sensitive. Hygroscopic. Incompatible with bases, oxidizing agents and reducing agents.
RUO – Research Use Only
General References:
- Can behave as a sulfide ion equivalent, e.g. the Cu-catalyzed reaction with aryl iodides leads to the diaryl sulfide, with loss of acrylic acid (reverse Michael): Synthesis, 523 (1989):
- In the presence of a free-radical initiator, acts as a chain transfer reagent in the telomerization of short oligomers for biotechnological applications: J. Polym. Sci. A, 37, 2977 (1999).
- For a brief review of the reagent, see: Synlett, 1368 (2002).
- Balamurugan, A.; Lee, H. Water-Soluble Polymeric Probes for the Selective Sensing of Mercury Ion: pH-Driven Controllable Detection Sensitivity and Time. Macromolecules 2015, 48 (4), 1048-1054.
- Wu, M.; Algar, W. R. Acceleration of Proteolytic Activity Associated with Selection of Thiol Ligand Coatings on Quantum Dots. ACS Appl. Mater. Interfaces 2015, 7 (4), 2535-2545.