Search Thermo Fisher Scientific
Thermo Scientific Chemicals
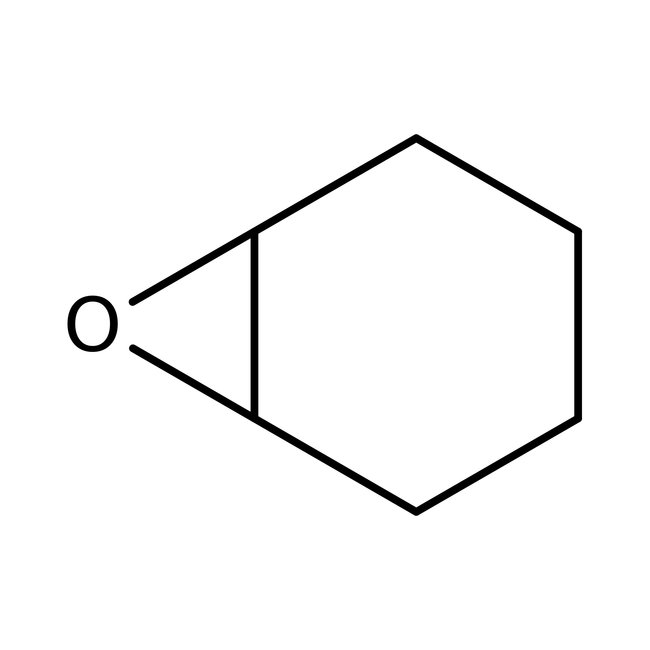
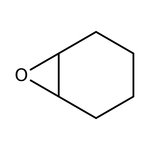
Cyclohexene oxide, 98+%, Thermo Scientific Chemicals
CAS: 286-20-4 | C6H10O | 98.145 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13185.36 | 500 g |
Catalog number ALFA13185.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS5619-04-5
IUPAC Namehydrogen methyl 2-amino-3-hydroxypropanoate chloride
Molecular FormulaC4H10ClNO3
InChI KeyNDBQJIBNNUJNHA-UHFFFAOYNA-N
SMILES[H+].[Cl-].COC(=O)C(N)CO
View more
Specifications Specification Sheet
Specification Sheet
Assay (Titration ex Chloride)≥98.0 to ≤102.0%
FormCrystals or powder or crystalline powder
Water Content (Karl Fischer Titration)≤1%
Appearance (Color)White to pale cream
It is an important raw material and intermediate used in organic synthesis, dyestuffs and in agrochemical industries. It is used in medicine. Cyclohexene oxide (epoxycyclohexane) is an useful monomer in polymerization and coating industry. It is used in the synthesis of alicyclic target materials including pharmaceuticals, perfumery and dyestuffs. It is used as a monomer in photopolymerizations, with carbonmonoxide to yield aromatic polycarbonates which has minimum impurities.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is an important raw material and intermediate used in organic synthesis, dyestuffs and in agrochemical industries. It is used in medicine. Cyclohexene oxide (epoxycyclohexane) is an useful monomer in polymerization and coating industry. It is used in the synthesis of alicyclic target materials including pharmaceuticals, perfumery and dyestuffs. It is used as a monomer in photopolymerizations, with carbonmonoxide to yield aromatic polycarbonates which has minimum impurities.
Solubility
Insoluble in water
Notes
Store at room temperature. Incompatible with oxidizing agents.
It is an important raw material and intermediate used in organic synthesis, dyestuffs and in agrochemical industries. It is used in medicine. Cyclohexene oxide (epoxycyclohexane) is an useful monomer in polymerization and coating industry. It is used in the synthesis of alicyclic target materials including pharmaceuticals, perfumery and dyestuffs. It is used as a monomer in photopolymerizations, with carbonmonoxide to yield aromatic polycarbonates which has minimum impurities.
Solubility
Insoluble in water
Notes
Store at room temperature. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Donald. J. Darensbourg .;Jason C. Yarbrough .; Cesar Ortiz.; Cindy C. Fang. Comparative Kinetic Studies of the Copolymerization of Cyclohexene Oxide and Propylene Oxide with Carbon Dioxide in the Presence of Chromium Salen Derivatives. In Situ FTIR Measurements of Copolymer vs Cyclic Carbonate Production.J. Am. Chem. Soc. 2003, 125 (25),7586-7591 .
- Stephan Mang.; Andrew I. Cooper.; M. Eamon Colclough.; Naren Chauhan.; Andrew B. Holmes. Copolymerization of CO2 and 1,2-Cyclohexene Oxide Using a CO2-Soluble Chromium Porphyrin Catalyst. Macromolecules. 2000, 33 (2),303-308 .
- A general method for the conversion of epoxides to cis-dichloro alkanes, with inversion at both centers, by reaction with triphenylphosphine dichloride, has been exemplified: Org. Synth. Coll., 6, 424 (1988).
- Rearrangement to cyclopentanecarboxaldehyde is catalyzed by LiBr on alumina, either by refluxing in toluene or in the gas phase: Synthesis, 394 (1988). An alternative isomerization to the allylic alcohol is induced by base catalysis. Optimized conditions use a mixture of KO-t-Bu and 2.5 equivalents of LDA: Tetrahedron, 46, 2411 (1990). For discussion of the mechanism, see: J. Org. Chem., 61, 820 (1996), and references therein. For a review of the rearrangement of epoxides to allylic alcohols, see: Org. React., 29, 345 (1983).
- A general method for the synthesis of ɑ-chloro carbonyl compounds is illustrated by the reaction of cylohexene oxide with the Swern reagent (DMSO/ oxalyl chloride) to give 2-chlorocyclohexanone in 93% yield: Tetrahedron, 51, 2467 (1995).