Search Thermo Fisher Scientific
Thermo Scientific Chemicals
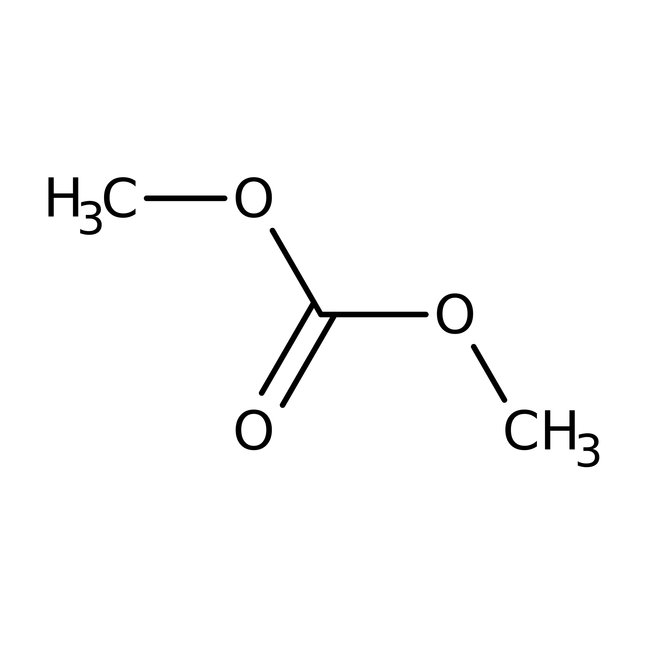
Dimethyl carbonate, 99%, Thermo Scientific Chemicals
CAS: 616-38-6 | C3H6O3 | 90.08 g/mol
Catalog number ALFA13104.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialDimethyl carbonate
CAS616-38-6
Health Hazard 1H225
Health Hazard 2GHS H Statement
H225
Highly flammable liquid and vapour.
H225
Highly flammable liquid and vapour.
Health Hazard 3P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P370+P378q-P501c
View more
Dimethyl carbonate is used as a solvent in organic synthesis and considered as a replacement for solvent like methyl ethyl ketone, tert-butyl acetate and parachlorobenzotrifluoride. It is involved as an intermediate in the preparation of diphenylcarbonate, which in turn is used as a key raw material for the synthesis of Bisphenol-A-polycarbonate. It is also used as a 'green' methylating agent involved in the methylation of aniline, phenols and carboxylic acids. It can be used as a fuel additive due to its high oxygen content. It also finds applications related to supercapacitors and lithium batteries.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Dimethyl carbonate is used as a solvent in organic synthesis and considered as a replacement for solvent like methyl ethyl ketone, tert-butyl acetate and parachlorobenzotrifluoride. It is involved as an intermediate in the preparation of diphenylcarbonate, which in turn is used as a key raw material for the synthesis of Bisphenol-A-polycarbonate. It is also used as a ′green′ methylating agent involved in the methylation of aniline, phenols and carboxylic acids. It can be used as a fuel additive due to its high oxygen content. It also finds applications related to supercapacitors and lithium batteries.
Solubility
Miscible with alcohol and ether. Immiscible with water.
Notes
Air and moisture sensitive. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents, strong acids and strong bases.
Dimethyl carbonate is used as a solvent in organic synthesis and considered as a replacement for solvent like methyl ethyl ketone, tert-butyl acetate and parachlorobenzotrifluoride. It is involved as an intermediate in the preparation of diphenylcarbonate, which in turn is used as a key raw material for the synthesis of Bisphenol-A-polycarbonate. It is also used as a ′green′ methylating agent involved in the methylation of aniline, phenols and carboxylic acids. It can be used as a fuel additive due to its high oxygen content. It also finds applications related to supercapacitors and lithium batteries.
Solubility
Miscible with alcohol and ether. Immiscible with water.
Notes
Air and moisture sensitive. Keep container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- Conversion of a ketone to its methoxycarbonyl derivative has been used to change the preferred site of chlorination to the less-substituted carbon atom: Synthesis, 188 (1987):
- Grignard reagents react in THF to give methyl esters, providing a high-yield, one-pot synthesis of carboxylic esters from alkyl or aryl halides: Synth. Commun., 20, 3273 (1990).
- Useful alkylating agent. Although less reactive, dimethyl carbonate has the advantage of much lower toxicity than the more usual sulfate or iodide. In the presence of K2CO3 and 18-crown-6, alcohols, phenols, thiols, imidazoles, etc. can be methylated: Synthesis, 382 (1986). For K2CO3 promoted alkylation of active methylene compounds at 180-220° (pressure), see: Rec. Trav. Chim., 115, 256 (1996); Org. Synth., 76, 169 (1998). Other dialkyl carbonates behave similarly.
- For other reactions of dialkyl carbonates, see Diethyl carbonate, A12477.
- Nale, D. B.; Bhanage, B. M. Copper-catalyzed efficient synthesis of a 2-benzimidazolone scaffold from 2-nitroaniline and dimethyl carbonate via a hydrosilylation reaction. Green Chem. 2015, 17 (4), 2480-2486.
- Earle, M. J.; Noe, M.; Perosa, A.; Seddon, K. R. Improved synthesis of tadalafil using dimethyl carbonate and ionic liquids. RSC Adv. 2014, 4 (3), 1204-1211.

.png-150.jpg)
