Search Thermo Fisher Scientific
Thermo Scientific Chemicals
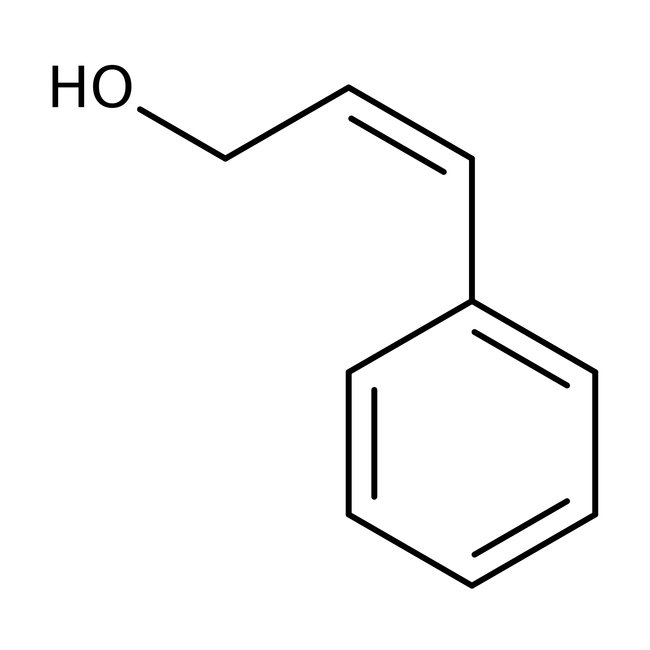
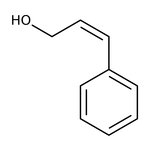
Cinnamyl alcohol, 98%, Thermo Scientific Chemicals
CAS: 104-54-1 | C9H10O | 134.178 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13025.30 | 250 g |
Catalog number ALFA13025.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialCinnamyl alcohol
CAS104-54-1
Health Hazard 1H302-H317-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H317
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
H302-H315-H319-H317
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause an allergic skin reaction.
Health Hazard 3P261-P264b-P270-P271-P272-P280g-P301+P312-P302+P352-P304+P340-P312-P330-P333+P313-P363-P501c
View more
Cinnamyl alcohol is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. It is also used in perfumery and as well as a deodorant.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cinnamyl alcohol is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. It is also used in perfumery and as well as a deodorant.
Solubility
Soluble in water 1.8 g/L (20°C).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Incompatible with strong oxidizing agents.
Cinnamyl alcohol is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuff. It is also used in perfumery and as well as a deodorant.
Solubility
Soluble in water 1.8 g/L (20°C).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Stable under recommended storage conditions. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- E Armengol.; ML Cano.; A Corma.; H García. Mesoporous aluminosilicate MCM-41 as a convenient acid catalyst for Friedel-Crafts alkylation of a bulky aromatic compound with cinnamyl alcohol. Journal of the Chemical Society, Chemical Communications.19957 (1), 519-520.
- RL Mansell.; GG Gross.; J Stöckigt.; H Franke.; MH Zenk. Purification and properties of cinnamyl alcohol dehydrogenase from higher plants involved in lignin biosynthesis. Phytochemistry. 19747 (1), 519-520.
- Protection of carboxylic acids as cinnamyl esters has been reported: J. Chem. Soc., Chem. Commun., 707 (1996). They can be cleaved under neutral conditions with Hg(II) or KSCN: Tetrahedron Lett., 2081 (1977).
- Allylic alcohols react with primary or secondary amines in the presence of SnCl2 and a Pd(0) catalyst to give allylic amines regioselectively: Chem. Lett., 1121 (1995):