Search Thermo Fisher Scientific
Thermo Scientific Chemicals
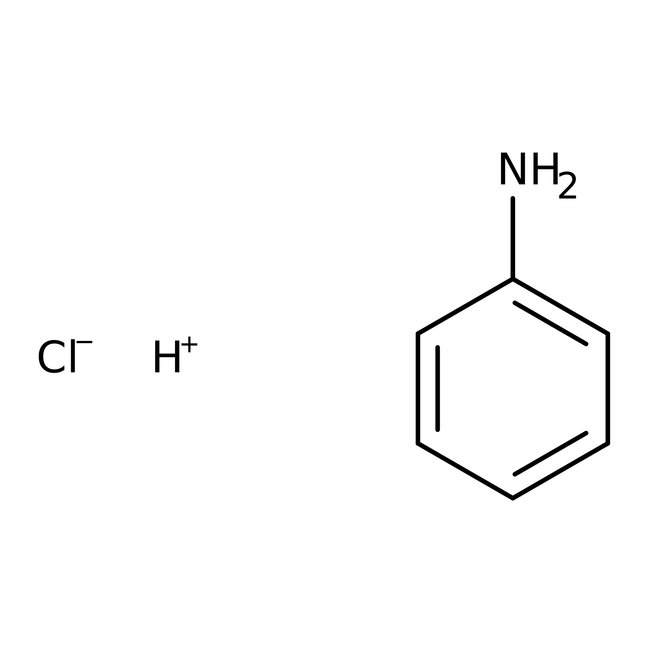
Aniline hydrochloride, 99%, Thermo Scientific Chemicals
CAS: 142-04-1 | C6H8ClN | 129.59 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13024.22 | 100 g |
Catalog number ALFA13024.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS107-06-2
IUPAC Name1,2-dichloroethane
Molecular FormulaC2H4Cl2
InChI KeyWSLDOOZREJYCGB-UHFFFAOYSA-N
SMILESClCCCl
View more
Specifications Specification Sheet
Specification Sheet
Free acid (HCl)=<0.01 %
Color scale=<25 APHA
Refractive index1.4440 to 1.4460 (20°C, 589 nm)
Appearance (Form)Clear liquid
Infrared spectrumConforms
View more
Aniline hydrochloride was used in the preparation of polyaniline coated poly(styrene-co-styrene sulfonate) nanoparticles. It was used to study the induction of Nei-like DNA glycosylases (NEIL1/2)-mediated base excision repair(BER) in rat spleen and 8-oxoguanine glycosylase 1-mediated BER due to aniline exposure. It is used in printing inks, clothing marking inks, paints and paint removers. It is also used as a stove polisher and shoe polisher, as well as in the synthesis of dyes, crayons and antioxidants.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Aniline hydrochloride was used in the preparation of polyaniline coated poly(styrene-co-styrene sulfonate) nanoparticles. It was used to study the induction of Nei-like DNA glycosylases (NEIL1/2)-mediated base excision repair(BER) in rat spleen and 8-oxoguanine glycosylase 1-mediated BER due to aniline exposure. It is used in printing inks, clothing marking inks, paints and paint removers. It is also used as a stove polisher and shoe polisher, as well as in the synthesis of dyes, crayons and antioxidants.
Solubility
Soluble in water (1070 g/L).
Notes
It is light sensitive and hygroscopic. Incompatible with oxidizing agents.
Aniline hydrochloride was used in the preparation of polyaniline coated poly(styrene-co-styrene sulfonate) nanoparticles. It was used to study the induction of Nei-like DNA glycosylases (NEIL1/2)-mediated base excision repair(BER) in rat spleen and 8-oxoguanine glycosylase 1-mediated BER due to aniline exposure. It is used in printing inks, clothing marking inks, paints and paint removers. It is also used as a stove polisher and shoe polisher, as well as in the synthesis of dyes, crayons and antioxidants.
Solubility
Soluble in water (1070 g/L).
Notes
It is light sensitive and hygroscopic. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- M.Khan Firoze; Bhupendra S.Kaphalia; Paul J.Boor; G.A.S.Ansari. Subchronic toxicity of aniline hydrochloride in rats. Archives of Environmental Contamination and Toxicology. 1993, 24, (3),368-374
- A. Barnes; A.Despotakis; P.V.Wright; T.C.P.Wong; B.Chambers; A.P.Anderson. Control of conductivity at microwave frequencies in a poly(aniline hydrochloride)-silver-polymer electrolyte composite material. Electronics Letters. 1996, 32, (4),358-359
- A convenient method has been described for generating aryl isocyanates from aniline hydrochlorides and oxalyl chloride, via thermolysis of the intermediate oxamic chloride: Tetrahedron Lett., 45, 4769 (2004).