Search Thermo Fisher Scientific
Thermo Scientific Chemicals
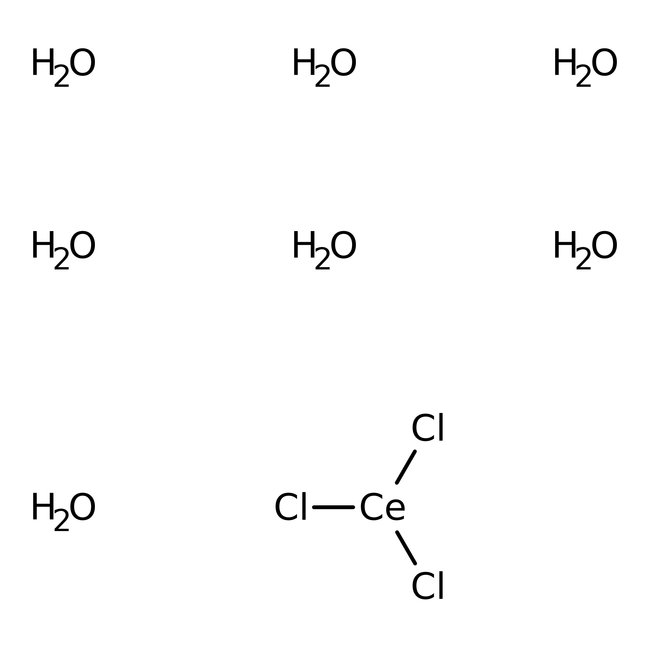
Cerium(III) chloride heptahydrate, 99%, Thermo Scientific Chemicals
CAS: 18618-55-8 | CeCl3·7H2O
Catalog number ALFA12947.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialCerium(III) chloride heptahydrate
CAS18618-55-8
Health Hazard 1H314
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P260-P264b-P280-P301+P330+P331-P303+P361+P353-P304+P340-P305+P351+P338-P310-P363-P501c
View more
Cerium(III) chloride heptahydrate is used in the preparation of allylsilanes from esters. It is used as a reducing agent in organic synthesis in place of sodium borohydride. In Luche reaction, carvone gives selectively allylic alcohol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cerium(III) chloride heptahydrate is used in the preparation of allylsilanes from esters. It is used as a reducing agent in organic synthesis in place of sodium borohydride. In Luche reaction, carvone gives selectively allylic alcohol.
Solubility
Soluble in alcohol and acetone. Slightly soluble in tetrahydrofuran. Insoluble in cold water.
Notes
Air sensitive. Hygroscopic. Incompatible with strong acids and strong oxidizing agents.
Cerium(III) chloride heptahydrate is used in the preparation of allylsilanes from esters. It is used as a reducing agent in organic synthesis in place of sodium borohydride. In Luche reaction, carvone gives selectively allylic alcohol.
Solubility
Soluble in alcohol and acetone. Slightly soluble in tetrahydrofuran. Insoluble in cold water.
Notes
Air sensitive. Hygroscopic. Incompatible with strong acids and strong oxidizing agents.
RUO – Research Use Only
General References:
- Reagent for selective cleavage of MEM ethers (see 2-Methoxyethoxymethyl chloride, L01050 ) under mild conditions: Org. Lett., 3, 1149 (2001).
- Useful in modifying the reactivity of Sodium borohydride, 35788 , allowing selective reduction of ketones in the presence of aldehydes: J. Am. Chem. Soc., 101, 5848 (1979), and giving increased selectivity for 1,2-reduction of enones to allylic alcohols: J. Chem. Soc., Chem. Commun., 601 (1978); cyclohexenones can be reduced in an alkyl alcohol to give the alkyl allylic ether in high yield: Pol. J. Chem., 69, 1655 (1995).
- With a catalytic amount of NaI in acetonitrile, dioxolanes (ethylene acetals) are deprotected to the parent carbonyl compounds: J. Org. Chem., 62, 4183 (1997), as are 4-methoxybenzyl ethers: J. Org. Chem., 64, 5696 (1999), and allyl ethers: Tetrahedron Lett., 40, 7293 (1999), to the parent alcohols. With a stoichiometric amount of NaI, alcohols can be converted to alkyl iodides: J. Org. Chem., 65, 2830 (2000), aryl alkyl ethers undergo dealkylation to phenols: Chem. Lett., 738 (2000), and selective deprotection of N-Boc protected tert-butyl amino acid esters can be achieved: J. Org. Chem., 66, 4430 (2001). Also deprotects the tert-butyl ethers of alcohols: Adv. Synth. Catal., 348, 905 (2006). For a review of the CeCl3·nH2OaI system,as an efficient, water-tolerant Lewis Acid promoter in organic synthesis, see: Synlett, 2101 (2003).
- In combination with Zn in acetonitrile, promotes the Reformatsky reaction of Ethyl bromofluoroacetate, B21579 : J. Org. Chem., 67, 72 (2002).
- For preparation of the anhydrous reagent and its use in combination with organolithium or Grignard reagents to suppress side reactions with carbonyl compounds, see: Org. Synth., 76, 228 (1998).
- For a brief feature on uses of this reagent, see: Synlett, 1935 (2002).

.png-150.jpg)
