Search Thermo Fisher Scientific
Thermo Scientific Chemicals
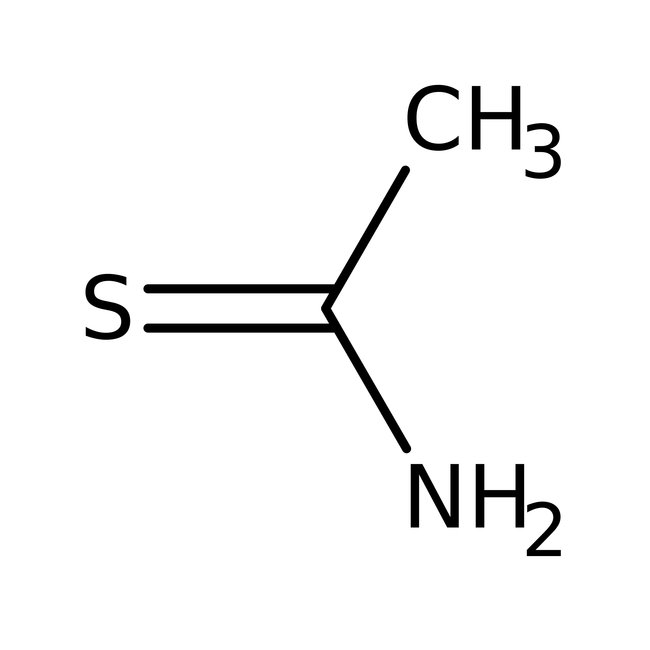
Thioacetamide, 98%, Thermo Scientific Chemicals
CAS: 62-55-5 | C2H5NS | 75.13 g/mol
Catalog number ALFA12926.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or MaterialThioacetamide
CAS62-55-5
Health Hazard 1H302-H315-H319-H336-H351-H373
Health Hazard 2GHS H Statement
H350-H302-H315-H319
H350-H302-H315-H319
Health Hazard 3P201-P202-P260-P264b-P270-P271-P280i-P281-P301+P312-P302+P352-P304+P340-P305+P351+P338-P308+P313-P330-P332+P313-P362-P501c
View more
Thioacetamide is used in qualitative inorganic analysis as an in-situ source for sulfide ions. It is used in analytical chemistry as a source of hydrogen sulfide. It is an additive in enantioselective reduction of beta-keto esters with immobilized bakers yeast. It is also used as a vulcanizing agent of polymer and a crosslinking agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Thioacetamide is used in qualitative inorganic analysis as an in-situ source for sulfide ions. It is used in analytical chemistry as a source of hydrogen sulfide. It is an additive in enantioselective reduction of beta-keto esters with immobilized bakers yeast. It is also used as a vulcanizing agent of polymer and a crosslinking agent.
Solubility
Soluble in ethanol, benzene and petroleum ether. Slightly soluble in water and ether.
Notes
Incompatible with strong oxidizing agents, strong acids and strong bases.
Thioacetamide is used in qualitative inorganic analysis as an in-situ source for sulfide ions. It is used in analytical chemistry as a source of hydrogen sulfide. It is an additive in enantioselective reduction of beta-keto esters with immobilized bakers yeast. It is also used as a vulcanizing agent of polymer and a crosslinking agent.
Solubility
Soluble in ethanol, benzene and petroleum ether. Slightly soluble in water and ether.
Notes
Incompatible with strong oxidizing agents, strong acids and strong bases.
RUO – Research Use Only
General References:
- You, M. S.; Lim, C. S.; Kwon, D. H.; Heo, J. H.; Im, S. H.; Chae, K. J. Oxide-free Sb2S3 sensitized solar cells fabricated by spin and heat-treatment of Sb (III)(thioacetamide)2Cl3. Org. Electron. 2015, 21, 155-159.
- Wei, D. D.; Wang, J. S.; Li, M. H.; Guo, P. P.; Dong, G.; Yang, M. H.; Kong, L. Y. A pilot study of the onset of hepatic encephalopathy (OHE) in mice induced by thioacetamide and the protective effect of taurine by holistic metabolic characterization. Metabolomics 2015, 11 (3), 559-570.