Search Thermo Fisher Scientific
Thermo Scientific Chemicals
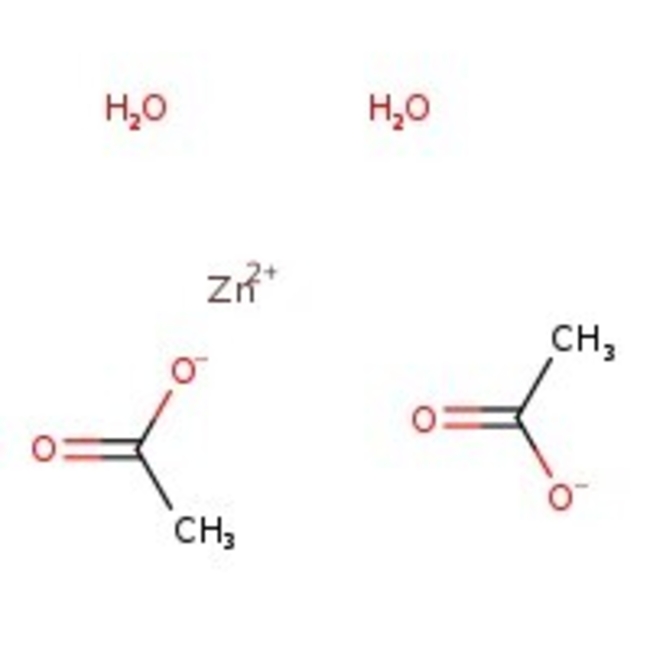
Zinc acetate dihydrate, 97+%, Thermo Scientific Chemicals
Catalog number ALFA12909.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS590-46-5
IUPAC Name(carboxymethyl)trimethylazanium chloride
Molecular FormulaC5H12ClNO2
InChI KeyHOPSCVCBEOCPJZ-UHFFFAOYSA-N
SMILES[Cl-].C[N+](C)(C)CC(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms (identification A)
Titration with HClO498.0 to 100.5 % (On anhydrous basis)
Residue after ignition=<0.1 %
Appearance (Form)Powder or crystals
Appearance (Color)Colorless to white
View more
Zinc acetate dihydrate is used in the preparation of layered Zn-arylphosphonates with potential application in sorption, ion exchange or catalysis and zinc sulfide nanoparticles coated on silica particles. It is used in dietary and medical applications. It is also used as lozenges for treating the common cold. It is used as a seeding material to grow zinc oxide nanorods on the microfibers of polyethylene terephthalate fabric. In addition, it finds application as wood preservation, production of other zinc salts, ethylene acetate manufacture, and an analytical reagent in various industries. As a plating inhibitor, it is used in nuclear power plant.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Solubility
Soluble in water and alcohol.
Notes
Incompatible with zinc salts, alkalies-their carbonates, oxalates, phosphates and sulfides.
Soluble in water and alcohol.
Notes
Incompatible with zinc salts, alkalies-their carbonates, oxalates, phosphates and sulfides.
RUO – Research Use Only
General References:
- Samsuri, S. A. M.; Rahman, M. Y. A.; Umar, A. A.; Salleh, M. M. Effect of zinc acetate dihydrate precursor concentration on the properties of TiO2-ZnO core-shell nanograss hetero-structure. J. Alloys Compd. 2015, 623, 460-465.
- Oh, S.; Jang, I.; Oh, S. G.; Im, S. S. Effect of ZnO nanoparticle morphology and post-treatment with zinc acetate on buffer layer in inverted organic photovoltaic cells. Sol. Energy 2015, 114, 32-38.