Search Thermo Fisher Scientific
Thermo Scientific Chemicals
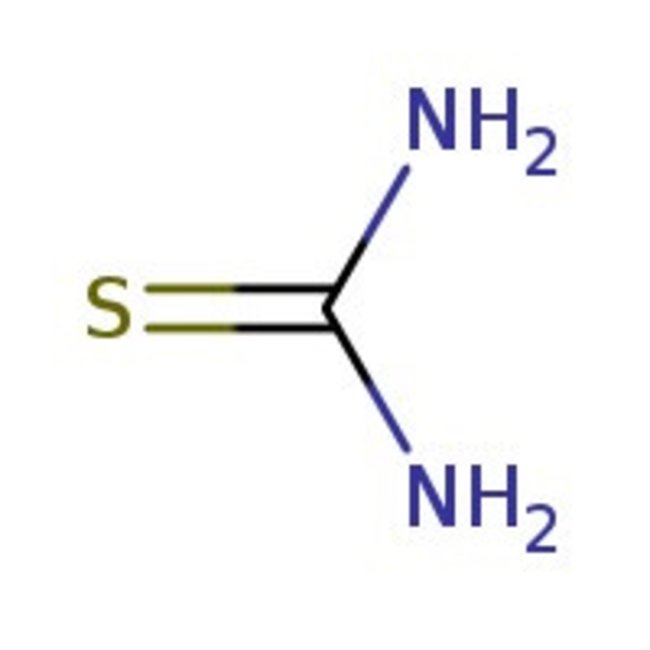
Thiourea, 99%, Thermo Scientific Chemicals
CAS: 62-56-6 | CH4N2S | 76.12 g/mol
Catalog number ALFA12828.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS73-24-5
IUPAC Name7H-purin-6-amine
Molecular FormulaC5H5N5
InChI KeyGFFGJBXGBJISGV-UHFFFAOYSA-N
SMILESNC1=C2NC=NC2=NC=N1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream
FormPowder
Assay (Non-aqueous acid-base Titration)≥98.5 to ≤101.5%
Identification (FTIR)Conforms
Reagent for organic synthesisUsed as a reagent for organic synthesis. Thiourea is a photographic fixative, and used in manufacture of resins. It acts as a catalyst for asymmetric reactions. It plays an essential role as a catalyst for highly enantio- and diastereoselective additions reaction of oxindoles to nitroolefins. It is also useful to improve the productivity of mung bean.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Reagent for organic synthesisUsed as a reagent for organic synthesis. Thiourea is a photographic fixative, and used in manufacture of resins. It acts as a catalyst for asymmetric reactions. It plays an essential role as a catalyst for highly enantio- and diastereoselective additions reaction of oxindoles to nitroolefins. It is also useful to improve the productivity of mung bean.
Solubility
Soluble in water.
Notes
Stable. Incompatible with strong acids. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Handle and store under inert gas.
Reagent for organic synthesisUsed as a reagent for organic synthesis. Thiourea is a photographic fixative, and used in manufacture of resins. It acts as a catalyst for asymmetric reactions. It plays an essential role as a catalyst for highly enantio- and diastereoselective additions reaction of oxindoles to nitroolefins. It is also useful to improve the productivity of mung bean.
Solubility
Soluble in water.
Notes
Stable. Incompatible with strong acids. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Handle and store under inert gas.
RUO – Research Use Only
General References:
- Reagent for the conversion of alkyl halides to thiols by base hydrolysis of the isothiouronium salts; see also N-Acetyl thiourea, B21198. Cleavage of isothiouronium salts with base and alkylation of the resulting thiolate has been used as a convenient synthesis of unsymmetrical sulfides: Synth. Commun., 14, 209 (1984).
- Epoxides are converted to episulfides: J. Org. Chem., 26, 3467 (1961). The 2,3-epoxy alcohols resulting from the Sharpless enantioselective epoxidation can be converted to the corresponding episulfides with retention at both centers, using Ti(O-i-Pr)4 as mediator: J. Org. Chem., 53, 4114 (1988).
- Widely used in heterocyclic syntheses, e.g. of thiazoles and pyrimidines.
- Has been used in a convenient synthesis of isothiocyanates from oximes via the nitrile oxide: Tetrahedron Lett., 34, 8283 (1993); see also Benzaldoxime, A12053:
- Bui, T.; Syed, S.; Barbas, C. F. Thiourea-Catalyzed Highly Enantio- and Diastereoselective Additions of Oxindoles to Nitroolefins: Application to the Formal Synthesis of (+) -Physostigmine. J. Am. Chem. Soc. 2009, 131 (25), 8758-8759.
- Takemoto, Y. Development of Chiral Thiourea Catalysts and Its Application to Asymmetric Catalytic Reactions. Chem. Pharm. Bull. 2010, 58 (5), 593-601.