Search Thermo Fisher Scientific
Thermo Scientific Chemicals
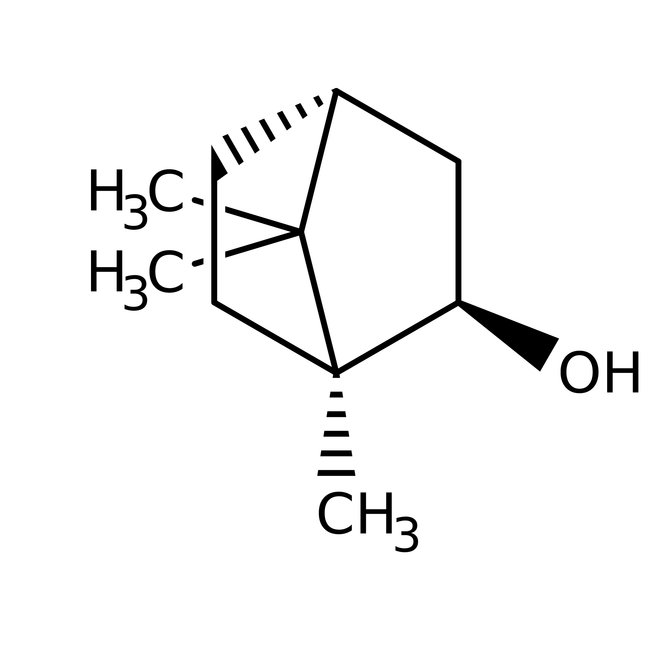
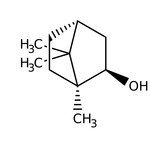
(-)-Borneol, 97+%, Thermo Scientific Chemicals
CAS: 464-45-9 | C10H18O | 154.25 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12684.18 | 50 g |
Catalog number ALFA12684.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS71989-38-3
IUPAC Name(2S)-3-[4-(tert-butoxy)phenyl]-2-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)propanoic acid
Molecular FormulaC28H29NO5
InChI KeyJAUKCFULLJFBFN-KSYWNVGFNA-N
SMILESCC(C)(C)OC1=CC=C(C[C@H](NC(=O)OCC2C3=C(C=CC=C3)C3=C2C=CC=C3)C(O)=O)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Assay (HPLC)>97.5%
(-)-Borneol is used to prepare its esters by reacting with acids. Its derivatives are used as chiral ligands in asymmetric synthesis. It is also used in flavors and perfumes. Further, it is used in traditional Chinese medicine as moxa. In addition to this, it is used as a component of many essential oils and also used as a natural insect repellent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
(-)-Borneol is used to prepare its esters by reacting with acids. Its derivatives are used as chiral ligands in asymmetric synthesis. It is also used in flavors and perfumes. Further, it is used in traditional Chinese medicine as moxa. In addition to this, it is used as a component of many essential oils and also used as a natural insect repellent.
Solubility
Soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin and tetralin. Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
(-)-Borneol is used to prepare its esters by reacting with acids. Its derivatives are used as chiral ligands in asymmetric synthesis. It is also used in flavors and perfumes. Further, it is used in traditional Chinese medicine as moxa. In addition to this, it is used as a component of many essential oils and also used as a natural insect repellent.
Solubility
Soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin and tetralin. Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Christoforides, E.; Mentzafos, D.; Bethanis, K. Structural studies of the inclusion complexes of the (+)- and (-)-borneol enantiomers in alpha- and beta-cyclodextrin. J. Incl. Phenom. Macro. Chem. 2015, 81 (1), 193-203.
- Marumoto, S.; Okuno, Y.; Miyamoto, Y.; Miyazawa, M. Biotransformation of (+)-(1R,2S,4R)-borneol and (-)-(1S,2R,4S)-borneol by Spodoptera litura (common cutworm) larvae. J. Mol. Catal. B: Enzym. 2015, 115, 160-167.