Search Thermo Fisher Scientific
Thermo Scientific Chemicals
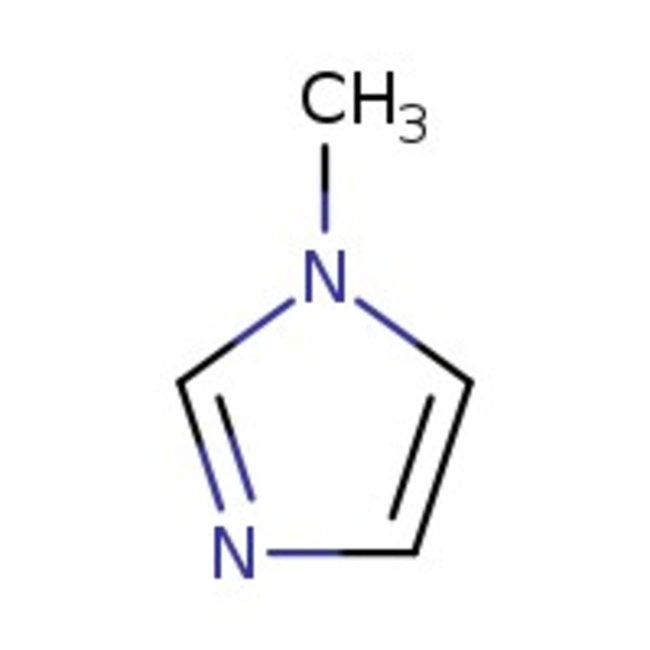
1-Methylimidazole, 99%, Thermo Scientific Chemicals
CAS: 616-47-7 | C4H6N2 | 82.11 g/mol
Catalog number ALFA12575.A3
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2 kg
Specifications
Chemical Name or Material1-Methylimidazole
CAS616-47-7
Health Hazard 1H227-H302-H311-H314-H335
Health Hazard 2GHS H Statement
H314-H302-H312-H227
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Combustible liquid.
H314-H302-H312-H227
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
1-Methylimidazole is used as a precursor for the synthesis of pyrrole-imidazole polyamides, ionic liquids such as 1-butyl-3-methylimidazolium hexafluorophosphate. It is actively involved in removing acid during the production of diethoxyphenylphosphine. It is used as an intermediate in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Methylimidazole is used as a precursor for the synthesis of pyrrole-imidazole polyamides, ionic liquids such as 1-butyl-3-methylimidazolium hexafluorophosphate. It is actively involved in removing acid during the production of diethoxyphenylphosphine. It is used as an intermediate in organic synthesis.
Solubility
Miscible with water.
Notes
Incompatible with carbon dioxide and strong oxidizing agents.
1-Methylimidazole is used as a precursor for the synthesis of pyrrole-imidazole polyamides, ionic liquids such as 1-butyl-3-methylimidazolium hexafluorophosphate. It is actively involved in removing acid during the production of diethoxyphenylphosphine. It is used as an intermediate in organic synthesis.
Solubility
Miscible with water.
Notes
Incompatible with carbon dioxide and strong oxidizing agents.
RUO – Research Use Only
General References:
- Useful base for peptide coupling, etc. See, e.g.: J. Chem. Soc., Chem. Commun., 2223 (1995). For peptide reagents, see Appendix 6.
- Undergoes lithiation at the 2-position. Reaction of the Li derivative with ketones followed by dehydration with acetic anhydride is a good route to 2-alkylideneimidazoles: Synthesis, 78 (1990). Likewise, reaction with benzonitrile gives the 2-benzoyl derivative, the carbonyl group of which undergoes Wittig methylenation: Synth. Commun., 20, 321 (1990).
- Reacts with acid chlorides, including chloroformates, to give N-acylimidazolium salts, which are useful reagents for the acylation of, for example, amino acids: Bull. Soc. Chim. Fr., 1021 (1973).
- Zhang, Y.; Yin, S. C.; Lu, J. M. N-Heterocyclic carbene-palladium(II)-1-methylimidazole complex catalyzed allyl-aryl coupling of allylic alcohols with arylboronic acids in neat water. Tetrahedron 2015, 71 (4), 544-549.
- Guan, J. T.; Song, X. M.; Zhang, Z. Y.; Wei, B. M.; Dai, Z. Q. Catalytic activity of 1-methylimidazole-based phosphine ligands in the palladium-catalyzed Suzuki coupling reaction. Appl. Organomet. Chem. 2015, 29 (2), 87-89.