Search Thermo Fisher Scientific
Thermo Scientific Chemicals
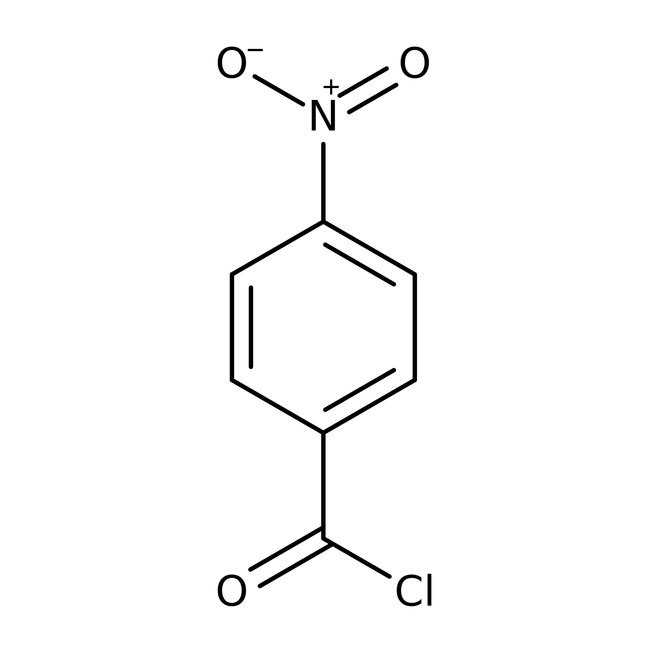
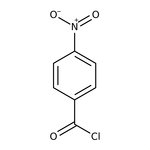
4-Nitrobenzoyl chloride, 98%, Thermo Scientific Chemicals
CAS: 122-04-3 | C7H4ClNO3 | 185.56 g/mol
Catalog number ALFA12543.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS87-69-4
IUPAC Name2,3-dihydroxybutanedioic acid
Molecular FormulaC4H6O6
InChI KeyFEWJPZIEWOKRBE-UHFFFAOYNA-N
SMILESOC(C(O)C(O)=O)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Colorless to white to almost white
Appearance (Form)Crystalline powder or crystals
Infrared spectrumConforms
Melting point168°C to 172°C
Loss on drying=<0.5 % (1 g, 105°C)
View more
4-Nitrobenzoyl chloride is used in the preparation of polysubstituted furanonaphthoquinoines. It is also involved in Michael addition, Henry reaction, O-alkylation and cycloaddition reactions. Further, it is employed as an intermediate in active pharmaceutical ingredient and dyes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Nitrobenzoyl chloride is used in the preparation of polysubstituted furanonaphthoquinoines. It is also involved in Michael addition, Henry reaction, O-alkylation and cycloaddition reactions. Further, it is employed as an intermediate in active pharmaceutical ingredient and dyes.
Solubility
Soluble in terahydrofuran, dichloromethane, chloroform and pyridine.
Notes
Moisture sensitive. Incompatible with water, alcohols, oxidizing agents and strong bases.
4-Nitrobenzoyl chloride is used in the preparation of polysubstituted furanonaphthoquinoines. It is also involved in Michael addition, Henry reaction, O-alkylation and cycloaddition reactions. Further, it is employed as an intermediate in active pharmaceutical ingredient and dyes.
Solubility
Soluble in terahydrofuran, dichloromethane, chloroform and pyridine.
Notes
Moisture sensitive. Incompatible with water, alcohols, oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- Reagent for the preparation of crystalline esters.
- Chen, W.; Feng, Y.; Zhang, M.; Wu, J.; Zhang, J.; Gao, X.; He, J.; Zhang, J. Homogeneous benzoylation of cellulose in 1-allyl-3-methylimidazolium chloride: Hammett correlation, mechanism and regioselectivity. RSC Adv. 2015, 5 (72), 58536-58542.
- Skácel, J.; Budka, J.; Eigner, V.; Lhoták, P. Regioselective Friedel-Crafts acylation of calix[4]arenes. Tetrahedron 2015, 71 (13), 1959-1965.