Search Thermo Fisher Scientific
Thermo Scientific Chemicals
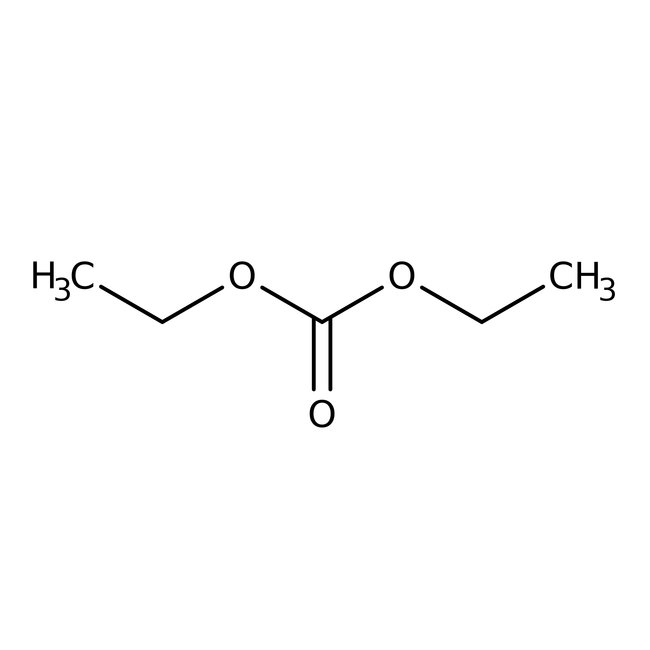
Diethyl carbonate, 99%, Thermo Scientific Chemicals
CAS: 105-58-8 | C5H10O3 | 118.13 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12477.AP | 500 mL |
Catalog number ALFA12477.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Chemical Identifiers
CAS25322-68-3
IUPAC Nameethane-1,2-diol
Molecular FormulaC2H6O2
InChI KeyLYCAIKOWRPUZTN-UHFFFAOYSA-N
SMILESOCCO
View more
Specifications Specification Sheet
Specification Sheet
Identification (FTIR)Conforms
FormPowder or flake
Water Content (Karl Fischer Titration)≤1.0%
Molecular Weight16,000-24,000
Viscosity2500-3500 mPa.s (50% aq. Soln. at 20?C)
View more
Diethyl carbonate is esters, beta-enamino esters, carbamates and unsymmetrical alkyl carbonates. It is an active component of electrolytes used in lithium which is used as a solvent for cellulose ethers, nitro cellulose, natural and synthetic resin and in erythromycin intramuscular injections. It is also used in the synthesis of 3-ethyl-4-methyl-5-phenyl-3H-oxazol-2-one, phenobarbital, pyrethrum batteries.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethyl carbonate is esters, beta-enamino esters, carbamates and unsymmetrical alkyl carbonates. It is an active component of electrolytes used in lithium which is used as a solvent for cellulose ethers, nitro cellulose, natural and synthetic resin and in erythromycin intramuscular injections. It is also used in the synthesis of 3-ethyl-4-methyl-5-phenyl-3H-oxazol-2-one, phenobarbital, pyrethrum batteries.
Solubility
Miscible with diehtyl ether, ethanol and chloroform.Immiscible with water.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong acids, oxidizing agents, strong bases and reducing agents.
Diethyl carbonate is esters, beta-enamino esters, carbamates and unsymmetrical alkyl carbonates. It is an active component of electrolytes used in lithium which is used as a solvent for cellulose ethers, nitro cellulose, natural and synthetic resin and in erythromycin intramuscular injections. It is also used in the synthesis of 3-ethyl-4-methyl-5-phenyl-3H-oxazol-2-one, phenobarbital, pyrethrum batteries.
Solubility
Miscible with diehtyl ether, ethanol and chloroform.Immiscible with water.
Notes
Store in cool place. Moisture sensitive. Incompatible with strong acids, oxidizing agents, strong bases and reducing agents.
RUO – Research Use Only
General References:
- Carboethoxylates carbanions derived from ketones, nitriles, esters, etc. For examples, see: Org. Synth. Coll., 4, 461 (1963); 5, 198 (1973); 6, 611 (1988). Arylacetic esters are converted to arylmalonic diesters: Org. Prep. Proced. Int., 6, 5 (1974). The dianions of carboxylic acids can be carboethoxylated with diethyl carbonate (or Ethyl chloroformate, L06311), to give monoesters of malonic acids: Tetrahedron Lett., 2721 (1974).
- For use as an alkylating agent for alcohols, phenols, thiols, etc., see Dimethyl carbonate, A13104.
- 1,3-Diols undergo dehydration to give oxetanes; see, e.g.: Tetrahedron Lett., 24, 5571 (1983):
- Srilakshmi, M.; Krishna, T. S.; Narendra, K.; Dey, R.; Ratnakar, A. Influence of alkyl group and temperature on excess thermodynamic properties of diethyl carbonate and their binary mixtures at 0.1 MPa. J. Mol. Liq. 2015, 211, 854-867.
- Li, F.; Li, H.; Wang, L.; He, P.; Cao, Y. Magnesium oxide nanosheets as effective catalysts for the synthesis of diethyl carbonate from ethyl carbamate and ethanol. Catal. Sci. Technol. 2015, 5 (2), 1021-1034.

.png-150.jpg)
