Search Thermo Fisher Scientific
Thermo Scientific Chemicals
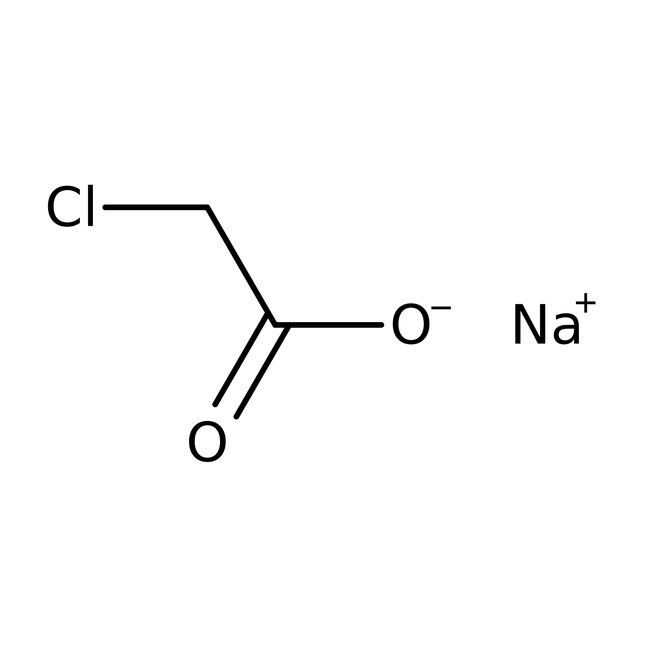
Sodium chloroacetate, 98%, Thermo Scientific Chemicals
CAS: 3926-62-3 | C2H2ClNaO2 | 116.476 g/mol
Catalog number ALFA12379.36
Quantity:
500 g
Chemical Identifiers
CAS75-58-1
Specifications Specification Sheet
Specification Sheet
FormPowder or crystalline powder
Assay from Suppliers CofA≥98.5% (NMR) (U.S. specification)
Water Content (Karl Fischer Titration)≤0.5%
Appearance (Color)White to pale yellow
Proton NMRConform to structure (U.S. specification)
View more
Sodium chloroacetate is used to prepare dyes and active pharmaceutical ingredients. It is also used as an odor agent, surface active agent and viscosity adjustor.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Sodium chloroacetate is used to prepare dyes and active pharmaceutical ingredients. It is also used as an odor agent, surface active agent and viscosity adjustor.
Solubility
Soluble in water, ether, chloroform, benzene and alcohol.
Notes
Incompatible with strong oxidizing agents.
Sodium chloroacetate is used to prepare dyes and active pharmaceutical ingredients. It is also used as an odor agent, surface active agent and viscosity adjustor.
Solubility
Soluble in water, ether, chloroform, benzene and alcohol.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- A convenient method for the conversion of arylamines to the corresponding N,N-diacetic acids is by lithiation followed by reaction with sodium chloroacetate. For discussion and limitations, see: Synthesis, 548 (1989).
- Ding, F.; Qian, X.; Zhang, Q.; Wu, H.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemically induced reversible formation of carboxymethyl chitin hydrogel and tunable protein release. New J. Chem. 2015, 39 (2), 1253-1259.
- Zhang, A.; Cheng, L.; Hong, S.; Yang, C.; Lin, Y. Preparation of anti-fouling silicone elastomers by covalent immobilization of carboxybetaine. RSC Adv. 2015, 5 (107), 88456-88463.