Search Thermo Fisher Scientific
Thermo Scientific Chemicals
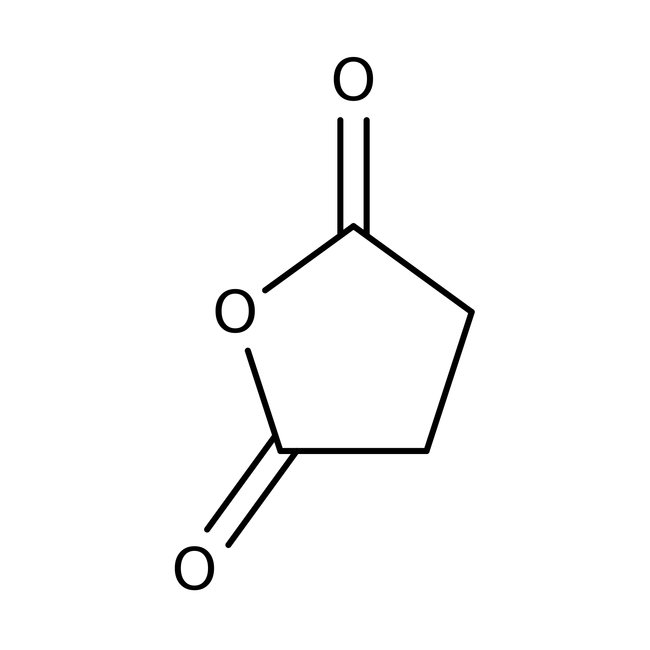
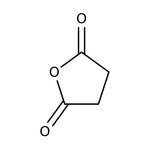
Succinic anhydride, 99%, Thermo Scientific Chemicals
CAS: 108-30-5 | C4H4O3 | 100.07 g/mol
Catalog number ALFA12245.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialSuccinic anhydride
CAS108-30-5
Melting Point118°C to 121°C
Boiling Point261°C
Health Hazard 1H302-H314-H317-H334
View more
Succinic anhydride is used as a sizing agent and curative for epoxy resin. It acts as an additive in paper production. It is utilized in the preparation of covalently cross-linked oxidized-alginate and N-succinyl-chitosan hydrogels. It serves as an intermediate for functionalized oxide surfaces on a chip and for polyester resins. Further, it finds use in cosmetics, pharmaceuticals and in coatings.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Succinic anhydride is used as a sizing agent and curative for epoxy resin. It acts as an additive in paper production. It is utilized in the preparation of covalently cross-linked oxidized-alginate and N-succinyl-chitosan hydrogels. It serves as an intermediate for functionalized oxide surfaces on a chip and for polyester resins. Further, it finds use in cosmetics, pharmaceuticals and in coatings.
Solubility
Soluble in chloroform.Soluble in chloroform, carbon tetrachloride and alcohol. Slightly soluble in ether. Insoluble in water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents, alcohols and amines.
Succinic anhydride is used as a sizing agent and curative for epoxy resin. It acts as an additive in paper production. It is utilized in the preparation of covalently cross-linked oxidized-alginate and N-succinyl-chitosan hydrogels. It serves as an intermediate for functionalized oxide surfaces on a chip and for polyester resins. Further, it finds use in cosmetics, pharmaceuticals and in coatings.
Solubility
Soluble in chloroform.Soluble in chloroform, carbon tetrachloride and alcohol. Slightly soluble in ether. Insoluble in water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents, alcohols and amines.
RUO – Research Use Only
General References:
- Friedel-Crafts reaction with arenes gives 3-aroylpropionic acids: Org. Synth. Coll., 2, 81 (1943). For a review of the Friedel-Crafts reactions of the anhydrides of dibasic acids, see: Org. React., 5, 229 (1949).
- For reaction with phosphoranes, in a route to 2,2-disubstituted cyclopentane-1,3-diones, see (1-Ethoxycarbonyl ethyl idene) triphenyl phosphorane, A15619.
- A method for protecting both H atoms of a primary amine consists of formation of the succinimide, followed by enolsilylation with Triisopropyl silyl trifluoromethanesulfonate, B21127, to form the 2,5-bis(triisopropylsiloxy)pyrroles. Deprotection can be effected by desilylation with dilute HCl, followed by hydrazinolysis: Tetrahedron Lett., 38, 2617 (1997).
- Peng, S.; Xue, L.; Leng, X.; Yang, R.; Zhang, G.; Hamaker, B. R. Slow Digestion Property of Octenyl Succinic Anhydride Modified Waxy Maize Starch in the Presence of Tea Polyphenols. J. Agric. Food. Chem. 2015, 63 (10), 2820-2829.
- Bello-Pérez, L. A.; Bello-Flores, C. A.; del Carmen Nuñez-Santiago, M.; Coronel-Aguilera, C. P.; Alvarez-Ramirez, J. Effect of the degree of substitution of octenyl succinic anhydride-banana starch on emulsion stability. Carbohydr. Polym. 2015, 132, 17-24.