Search Thermo Fisher Scientific
Thermo Scientific Chemicals
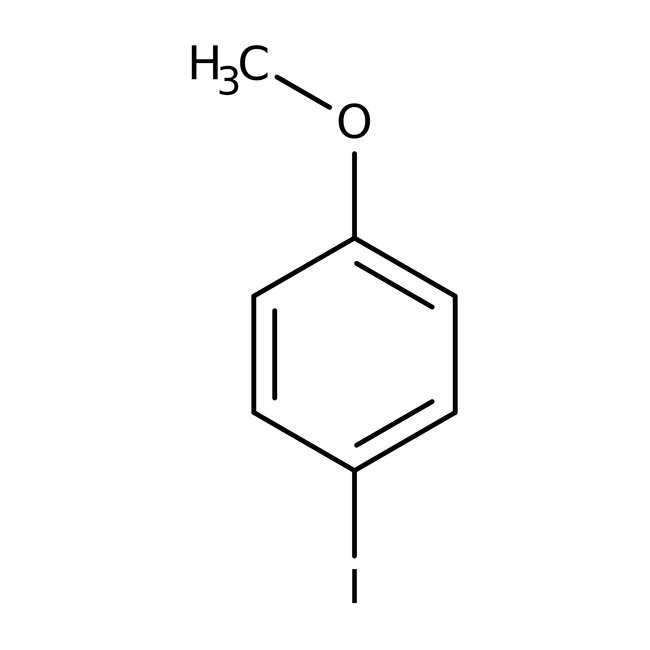
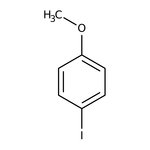
4-Iodoanisole, 98+%, Thermo Scientific Chemicals
CAS: 696-62-8 | C7H7IO | 234.036 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA12144.14 | 25 g |
Catalog number ALFA12144.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material4-Iodoanisole
CAS696-62-8
Health Hazard 1Warning
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3GHS P Statement
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
4-Iodoanisole is used in wide range of medicals industrial applications as well as in human and animal nutrition products, pharmaceutical intermediates, polarizing films for Liquid Crystal Display (LCD) chemicals. Iodine derivatives are also used as organic building blocks, analytical reagents.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Iodoanisole is used in wide range of medicals industrial applications as well as in human and animal nutrition products such as antiseptics and disinfectants, pharmaceutical intermediates, polarizing films for liquid crystal display [LCD] chemicals. Iodine derivatives are also used as organic building blocks, analytical reagents.
Solubility
Soluble in ethanol, ether, chloroform.
Notes
Light sensitive. Store in the dark. Store away from oxidizing agents, light.
4-Iodoanisole is used in wide range of medicals industrial applications as well as in human and animal nutrition products such as antiseptics and disinfectants, pharmaceutical intermediates, polarizing films for liquid crystal display [LCD] chemicals. Iodine derivatives are also used as organic building blocks, analytical reagents.
Solubility
Soluble in ethanol, ether, chloroform.
Notes
Light sensitive. Store in the dark. Store away from oxidizing agents, light.
RUO – Research Use Only
General References:
- Noel S. Murcia; Dennis G. Peters. Electroreductive carboxylation of halobenzenes. Production of p-anisic acid by reduction of p-iodoanisole at mercury in dimethylformamide saturated with carbon dioxide. J. Electroanal. Chem. 1992, 326 (1-2), 69-79.
- Gertrude Maud Robinson. LXXVII.—Experiments on the so-called migration of atoms and groups. Part I. The nitration of p-iodoanisole and other iodo-phenolic ethers. J. Chem. Soc., Trans. 1916, 109, 1078-1091.
- For use in the 'ligandless' Suzuki cross-coupling with arylboronic acids, see: Org. Synth., 75, 61 (1997).