Search Thermo Fisher Scientific
Thermo Scientific Chemicals
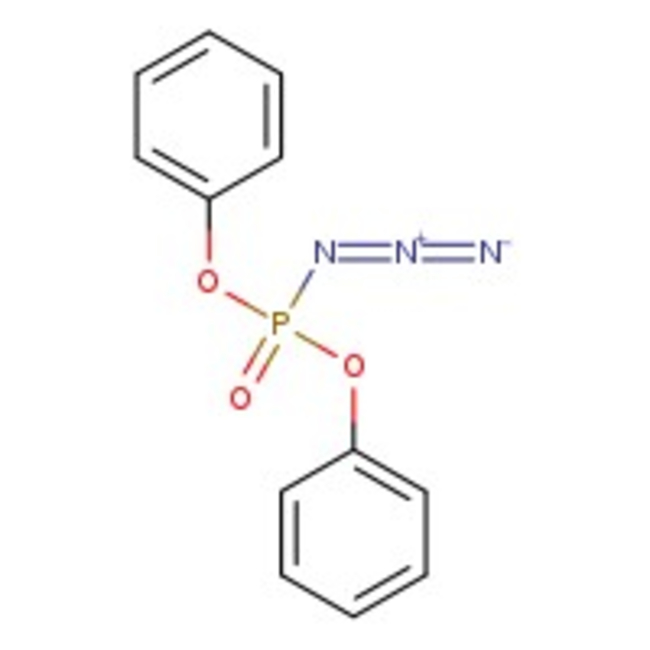
Diphenylphosphonic azide, 97%, Thermo Scientific Chemicals
Store cold
| Catalog Number | Quantity |
|---|---|
| ALFA12124.06 | 5 g |
Catalog number ALFA12124.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Chemical Identifiers
CAS620-40-6
IUPAC Nametribenzylamine
Molecular FormulaC21H21N
InChI KeyMXHTZQSKTCCMFG-UHFFFAOYSA-N
SMILESC(N(CC1=CC=CC=C1)CC1=CC=CC=C1)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Titration with HClO4>=99.0 % (On anhydrous substance)
Appearance (Form)Powder
Melting point90°C to 94°C
Water=<0.5 % (K.F.)
View more
Diphenylphosphonic azide acts as a reagent for the synthesis of peptides and phosphoramidates by reacting with amines. It is also used in the preparation of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement. It is involved in pseudohalogen replacement of the azido group by treatment with nucleophilic reagents, such as water, butanol, ammonia, and various amines. Further, it is used as a hydroazidation catalyst for preparation of organoazides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diphenylphosphonic azide acts as a reagent for the synthesis of peptides and phosphoramidates by reacting with amines. It is also used in the preparation of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement. It is involved in pseudohalogen replacement of the azido group by treatment with nucleophilic reagents, such as water, butanol, ammonia, and various amines. Further, it is used as a hydroazidation catalyst for preparation of organoazides.
Solubility
Immiscible with water.
Notes
Store in a cool place. Incompatible with acids and oxidizing agents.
Diphenylphosphonic azide acts as a reagent for the synthesis of peptides and phosphoramidates by reacting with amines. It is also used in the preparation of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement. It is involved in pseudohalogen replacement of the azido group by treatment with nucleophilic reagents, such as water, butanol, ammonia, and various amines. Further, it is used as a hydroazidation catalyst for preparation of organoazides.
Solubility
Immiscible with water.
Notes
Store in a cool place. Incompatible with acids and oxidizing agents.
RUO – Research Use Only
General References:
- Stable azide-transfer agent.
- In the presence of an amine, carboxylic acids are converted to acyl azides which undergo a modified Curtius reaction in the presence of an alcohol to give alkyl carbamates directly. With t-butanol, the resulting t-butyl carbamates can readily be converted to the free amines with mild acid. Malonic half-esters, e.g. Ethyl hydrogen malonate, A12627 , give ɑ-amino acid derivatives: J. Am. Chem. Soc., 94, 6203 (1972); Chem. Pharm. Bull., 22, 1398 (1974); J. Org. Chem., 49, 185 (1984):
- Use of the hindered base 1,8-Bis(dimethyl amino) naphthalene, L00313 , enables the isocyanate intermediates to be isolated: Synth. Commun., 23, 335 (1993). Application to ɑß-unsaturated acids provides a useful degradation to aldehydes with one C atom fewer by hydrolysis of the intermediate enamine. See, e.g.: Synth. Commun., 20, 589 (1990).
- N-protected amino acids are converted to acyl azides for use in a low racemization peptide coupling technique: J. Am. Chem. Soc., 94, 6203 (1972); Synthesis, 549 (1974); J. Org. Chem., 44, 3101 (1979); 52, 764 (1987). See Appendix 6. The method is also applicable to the coupling of carboxylic acids with thiols: J. Org. Chem., 39, 3302 (1974); Chem. Pharm. Bull., 25, 2423 (1977). Similarly, macrocyclic lactams have been prepared without high dilution by reaction of diacids with diamines: Tetrahedron Lett., 31, 6469 (1990).