Search Thermo Fisher Scientific
Thermo Scientific Chemicals
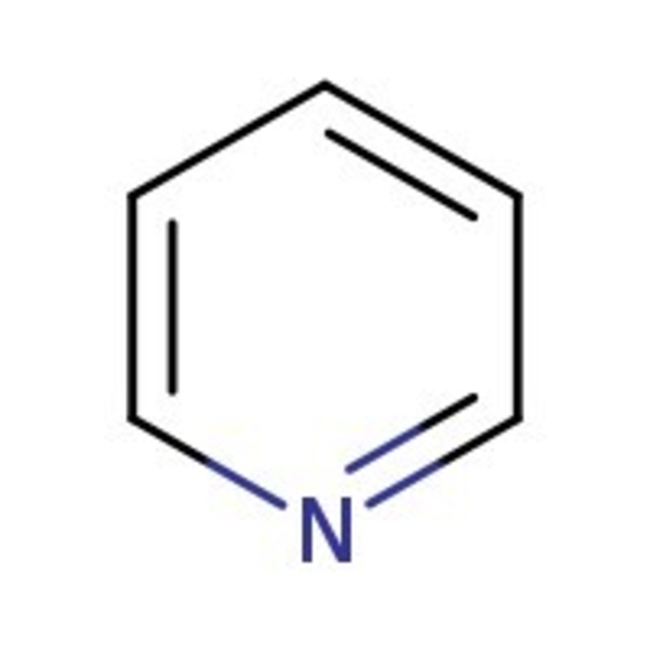
Pyridine, 99+%, Thermo Scientific Chemicals
CAS: 110-86-1 | C5H5N | 79.102 g/mol
Catalog number ALFA12005.0F
Price (MYR)
1,162.00
Quantity:
2500 mL
Price (MYR)
1,162.00
Specifications
Chemical Name or MaterialPyridine
Melting Point-42°C
CAS110-86-1
Health Hazard 1H225-H302+H312+H332-H315-H319
Health Hazard 2GHS H Statement
H225-H302-H312-H332
Highly flammable liquid and vapor.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
H225-H302-H312-H332
Highly flammable liquid and vapor.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
View more
As polar, basic, low-reactive solvent, precursor, and reagent, pyridine finds wide range of applications in pharmaceuticals, agrochemicals and in the manufacture of dyes and rubber. Further it is used in the textile industry to improve network capacity of cotton. Pyridine finds applications in specialty chemical reagents like Cornforth reagent (pyridinium dichromate, PDC), pyridinium chlorochromate (PCC), the Collins reagent (complex of chromium(VI) oxide with pyridine) and the Sarret reagent (complex of chromium(VI) oxide with pyridine). Pyridine is a widely used ligand in coordination chemistry. Pyridine-base adducts like pyridine-borane and pyridine-sulfur trioxide are widely used owing to their superior characteristics in their reactivity and solubility characteristics.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
As polar, basic, low-reactive solvent, precursor, and reagent, pyridine finds wide range of applications in pharmaceuticals, agrochemicals and in the manufacture of dyes and rubber. Further it is used in the textile industry to improve network capacity of cotton. Pyridine finds applications in specialty chemical reagents like Cornforth reagent (pyridinium dichromate, PDC), pyridinium chlorochromate (PCC), the Collins reagent (complex of chromium(VI) oxide with pyridine) and the Sarret reagent (complex of chromium(VI) oxide with pyridine). Pyridine is a widely used ligand in coordination chemistry. Pyridine-base adducts like pyridine-borane and pyridine-sulfur trioxide are widely used owing to their superior characteristics in their reactivity and solubility characteristics.
Solubility
Miscible with water, alcohol, ether, pet ether, and oils.
Notes
Pyridine is stable under normal temperatures and pressures. It is incompatible with strong oxidizing agents, and acids.
As polar, basic, low-reactive solvent, precursor, and reagent, pyridine finds wide range of applications in pharmaceuticals, agrochemicals and in the manufacture of dyes and rubber. Further it is used in the textile industry to improve network capacity of cotton. Pyridine finds applications in specialty chemical reagents like Cornforth reagent (pyridinium dichromate, PDC), pyridinium chlorochromate (PCC), the Collins reagent (complex of chromium(VI) oxide with pyridine) and the Sarret reagent (complex of chromium(VI) oxide with pyridine). Pyridine is a widely used ligand in coordination chemistry. Pyridine-base adducts like pyridine-borane and pyridine-sulfur trioxide are widely used owing to their superior characteristics in their reactivity and solubility characteristics.
Solubility
Miscible with water, alcohol, ether, pet ether, and oils.
Notes
Pyridine is stable under normal temperatures and pressures. It is incompatible with strong oxidizing agents, and acids.
RUO – Research Use Only
General References:
- Baumann, M.; Baxendale I. R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265-2319.
- Bull, J. A.; Mousseau, J. J.; Pelletier, G.; Charette, A. B. Synthesis of pyridine and dihydropyridine derivatives by regio- and stereoselective addition to N-activated pyridines. Chem. Rev. 2012, 112 (5), 2642-2713.
- Widely used as a base and nucleophilic catalyst in acylations, sulfonylations, etc. See also 4-(Dimethyl amino) pyridine, A13016.
- Reacts with thionyl chloride to give N-(4-pyridyl)pyridinium chloride hydrochloride, a useful intermediate for the preparation of 4-substituted pyridines by nucleophilic displacement. Thus, reaction with sulfite ion gives the sulfonate: Org. Synth. Coll., 5, 977 (1973). Similarly, pyridine can be activated to substitution at the 4-position by reaction with Ethyl chloroformate, L06311, or tert-Butyl dimethyl silyl trifluoromethanesulfonate, A12174. The acyl or silyl pyridinium salt then reacts with Grignards or organocuprates, to give 4-substituted dihydropyridines which can readily be rearomatized: Bull. Chem. Soc. Jpn., 57, 1994 (1984).
- For use in Cr(VI) oxidation reactions, see Chromium(VI) oxide, 12522.