Search Thermo Fisher Scientific
Thermo Scientific Chemicals
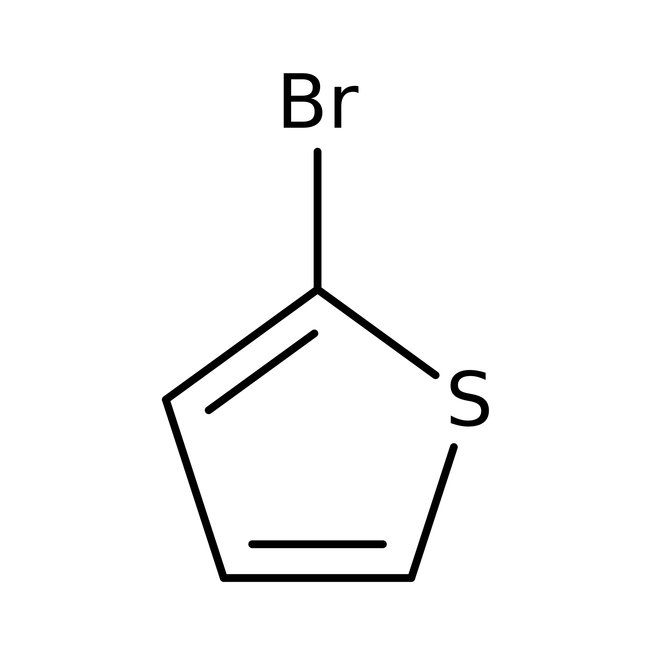
2-Bromothiophene, 98+%, Thermo Scientific Chemicals
CAS: 1003-09-4 | C4H3BrS | 163.03 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11959.18 | 50 g |
Catalog number ALFA11959.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS12125-02-9
IUPAC Nameammonium chloride
Molecular FormulaClH4N
InChI KeyNLXLAEXVIDQMFP-UHFFFAOYSA-N
SMILES[NH4+].[Cl-]
View more
Specifications Specification Sheet
Specification Sheet
Iron (Fe)=<10 ppm
Appearance (Color)White
Water=<0.1 % (K.F.)
Titration Argentometric>=99.0 %
Insoluble matter=<0.02 %
View more
2-Bromothiophene has been used in electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium per chlorate by cyclic voltammetry and controlled-potential electrolysis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Bromothiophene has been used in electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium per chlorate by cyclic voltammetry and controlled-potential electrolysis.
Solubility
Not miscible or difficult to mix in water.
Notes
Light Sensitive. Keep away from oxidizing agents. Store in dark.
2-Bromothiophene has been used in electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium per chlorate by cyclic voltammetry and controlled-potential electrolysis.
Solubility
Not miscible or difficult to mix in water.
Notes
Light Sensitive. Keep away from oxidizing agents. Store in dark.
RUO – Research Use Only
General References:
- Mubarak MS and Peters DG. Electrochemical Reduction of Mono- and Dihalothiophenes at Carbon Cathodes in Dimethylformamide. First Example of an Electrolytically Induced Halogen Dance.J. Org. Chem.,1996,61(23), 8074-8078.
- Peyron C, et al. First example of base-promoted tandem alkylation-bromination of 2-bromothiophene via halogen dance process: a remarkable temperature effect.Tetrahedron Lett.2005,46(19), 3315-3318.
- Sathyapalan A, et al. Novel self assembled monolayers of allyl phenyl thiophene ether as potential dielectric material for organic thin film transistors.Thin Solid Films,2008,516(16), 5645-5648.
- The bromo-substituent can be readily displaced by methoxide in methanol in the presence of a Cu(I) catalyst: Synth. Commun., 20, 213 (1990). For coupling reaction with Thiophene-2-thiol, B22642, in the presence of Cu2O, to give 2,2'-dithienyl sulfide, see: Org. Synth. Coll., 6, 558 (1988).
- Allylic alcohols are thienylated by means of a Heck-type, Pd-catalyzed coupling reaction; the products are ketones formed by rearrangement: Chem. Lett., 423 (1977). Coupling with iodoarenes can be induced at the 5-position by the use of a palladium(II) catalyst in the presence of silver nitrate and potassium fluoride, providing access to 5-aryl-2-bromothiophene derivatives, which may be further elaborated by reaction with the available 2-bromo-substituent: Org. Lett., 7, 5083 (2005).