Search Thermo Fisher Scientific
Thermo Scientific Chemicals
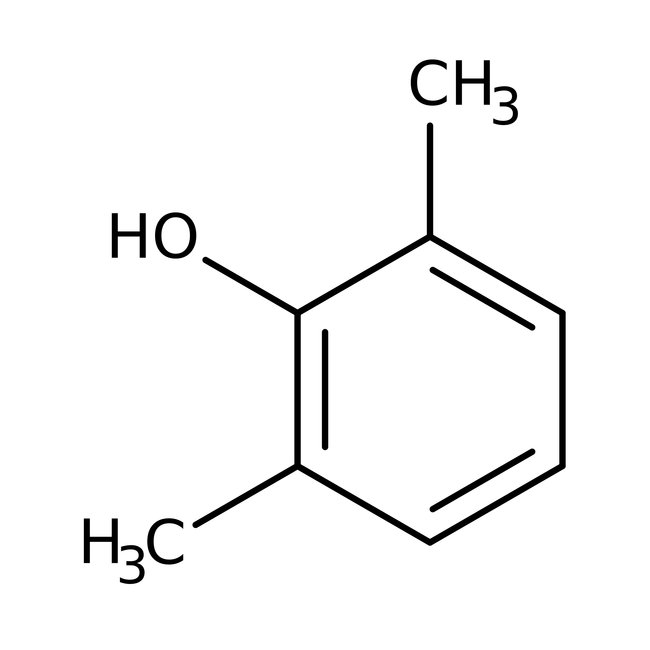
2,6-Dimethylphenol, 99%, Thermo Scientific Chemicals
CAS: 576-26-1 | C8H10O | 122.17 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11947.36 | 500 g |
Catalog number ALFA11947.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS1483-72-3
Specifications Specification Sheet
Specification Sheet
Infrared spectrumConforms
Appearance (Color)White to cream to yellow
Titration Argentometric>=96.0 %
Appearance (Form)Powder
2,6-dimethylphenol is used in the synthesis of anti-oxidant compounds due to the phenol moiety in the structure. In addition, this compound is used as a reactant in the synthesis of polyphenylene ether polymers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,6-dimethylphenol is used in the synthesis of anti-oxidant compounds due to the phenol moiety in the structure. In addition, this compound is used as a reactant in the synthesis of polyphenylene ether polymers.
Solubility
Soluble in water and chloroform.
Notes
Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from strong bases and oxidizing agent.
2,6-dimethylphenol is used in the synthesis of anti-oxidant compounds due to the phenol moiety in the structure. In addition, this compound is used as a reactant in the synthesis of polyphenylene ether polymers.
Solubility
Soluble in water and chloroform.
Notes
Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from strong bases and oxidizing agent.
RUO – Research Use Only
General References:
- Yasar Thewalim; Fredrik Aldaeus; Anders Colmsjö. Retention time prediction of compounds in Grob standard mixture for apolar capillary columns in temperature-programmed gas chromatography. Analytical and Bioanalytical Chemistry.2009,393(1), 327-334.
- Masahiro Ogata et. al. Antioxidant activity of propofol and related monomeric and dimeric compounds. Chemical & Pharmaceutical Bulletin,2005, 53(3), 344-346.
- O-Alkylation of this hindered phenol by alkyl halides has been achieved using NaOH with micellar catalysis by (1-Hexadecyl) trimethyl ammonium bromide, A15235: Tetrahedron, 44, 6677 (1988). Alkylation of the Li salt with MeI occurs preferentially on carbon to give the dimer of 2,6,6-trimethyl-2,4-cyclohexadienone: Org. Synth. Coll., 5, 1092 (1973).
- Addition of lithiated 2,6-dimethylphenyl esters of alkanoic acids to carbonyl compounds has been used in a highly diastereoselective synthesis of ß-hydroxy acids. In some cases, only one of the two possible diastereomers is formed. For list of examples, see: Org. Synth. Coll., 7, 190 (1990).