Search Thermo Fisher Scientific
Thermo Scientific Chemicals
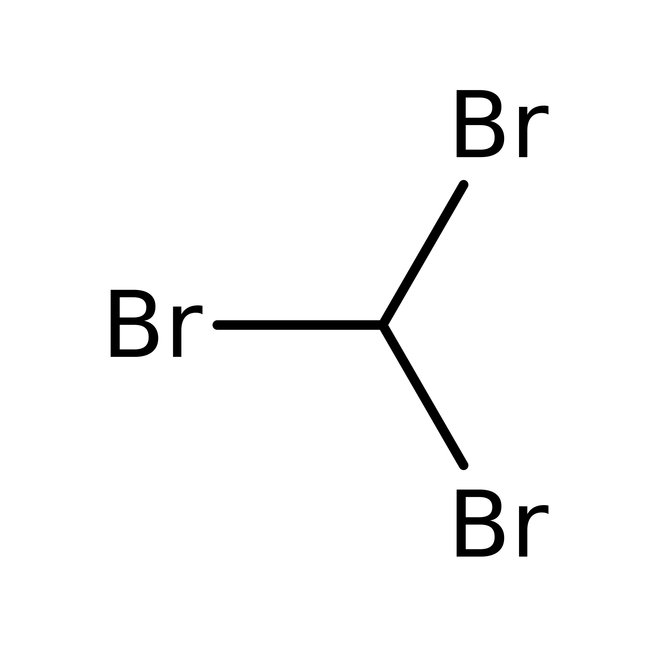
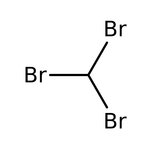
Bromoform, 96%, stab. with ethanol, Thermo Scientific Chemicals
Bromoform, 97%, CHBr3, CAS Number-75-25-2, tribromomethane, methenyl tribromide, methane, tribromo, tribrommethan, bromoforme, bromoformio, tribromometan, tribrommethaan, methyl tribromide, rcra waste number u225, 5000g, 1731048, 148 deg.C to 150 deg.C, CHEBI:38682, 2.81
Catalog number ALFA11904.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialBromoform
Name NoteStabilized with ethanol
CAS75-25-2
Health Hazard 1H302-H315-H319-H331
Health Hazard 2GHS H Statement
H351-H302-H332-H315-H319
Suspected of causing cancer.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
H351-H302-H332-H315-H319
Suspected of causing cancer.
Harmful if swallowed.
Harmful if inhaled.
Causes skin irritation.
Causes serious eye irritation.
View more
Bromoform is widely used as a solvent for waxes, oils and greases. It is utilized for mineral ore separation in geological tests. It is used as an intermediate in chemical synthesis as well as a laboratory reagent. It is the ingredient of fire-resistant chemicals and fluid gauges. It acts as a sedative and as cough reducing agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Bromoform is widely used as a solvent for waxes, oils and greases. It is utilized for mineral ore separation in geological tests. It is used as an intermediate in chemical synthesis as well as a laboratory reagent. It is the ingredient of fire-resistant chemicals and fluid gauges. It acts as a sedative and as cough reducing agent.
Solubility
Slightly soluble in water.
Notes
Light-sensitive. A lachrymator. Incompatible with chemically active metals and strong bases.
Bromoform is widely used as a solvent for waxes, oils and greases. It is utilized for mineral ore separation in geological tests. It is used as an intermediate in chemical synthesis as well as a laboratory reagent. It is the ingredient of fire-resistant chemicals and fluid gauges. It acts as a sedative and as cough reducing agent.
Solubility
Slightly soluble in water.
Notes
Light-sensitive. A lachrymator. Incompatible with chemically active metals and strong bases.
RUO – Research Use Only
General References:
- For examples of the generation of dibromocarbene, using Potassium tert-butoxide, A13947, as base, see: Org. Synth. Coll., 6, 187 (1988); 7, 200 (1990). Alternatively, reactions can be carried out with aqueous OH- and a phase-transfer catalyst such as Benzyl triethyl ammonium chloride, A13268: Org. Synth. Coll., 8, 223 (1993), or using crown ether catalysis: Org. Synth., 75, 98 (1997).
- Under phase-transfer conditions, allylic bromides undergo nucleophilic displacement by the tribromomethyl anion: J. Chem. Soc., Chem. Commun., 210 (1979).
- Wang, L.; Liu, P.; Zhang, S. Debromination and decomposition of bromoform by contact glow discharge electrolysis in an aqueous solution. Electrochim. Acta 2015, 165, 390-395.
- Liu, B.; Li, X. F.; Zhang, J.; Wang, M. T.; Zeng, R. J. Synthesis of novel spiro[cyclopropane-pyrrolizin] derivatives via Mg-mediated conjugate addition of bromoform. Res. Chem. Intermed. 2015, 41 (4), 2345-2352.