Search Thermo Fisher Scientific
Thermo Scientific Chemicals
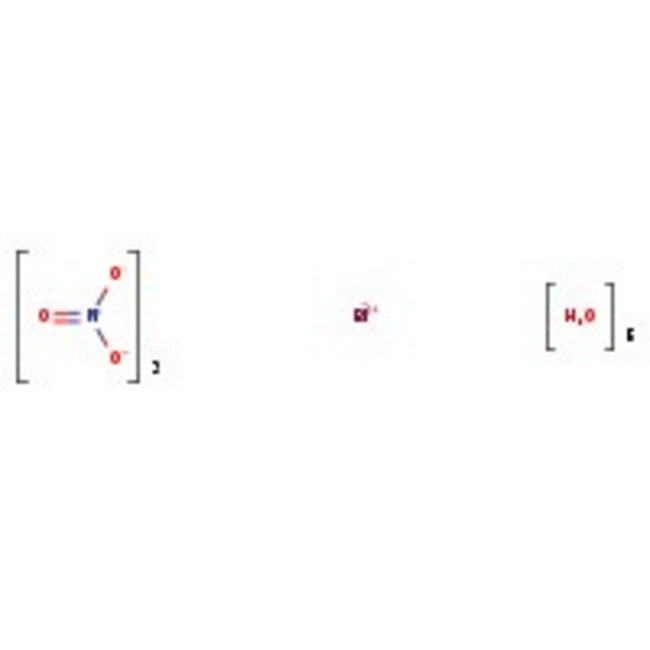
Bismuth(III) nitrate pentahydrate, 98%, Thermo Scientific Chemicals
CAS: 10035-06-0 | BiH10N3O14 | 485.07 g/mol
Catalog number ALFA11748.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialBismuth (III) nitrate pentahydrate
Melting Point30°C (decomposition)
CAS10035-06-0
Health Hazard 1H272-H315-H319-H335-H373
Health Hazard 2GHS H Statement
H272-H315-H319-H335
May intensify fire
oxidizer.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H272-H315-H319-H335
May intensify fire
oxidizer.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
View more
Bismuth(III) nitrate pentahydrate is an oxidant for a variety of 4-substituted Hantzsch 1,4-dihydropyridines. It also serves as a reagent for selective oxidation of sufides to sulfoxides. Further, it is involved in the preparation of Dragendorff reagent and used as a TLC stain. It is also used to prepare other bismuth compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Bismuth(III) nitrate pentahydrate is an oxidant for a variety of 4-substituted Hantzsch 1,4-dihydropyridines. It also serves as a reagent for selective oxidation of sufides to sulfoxides. Further, it is involved in the preparation of Dragendorff reagent and used as a TLC stain. It is also used to prepare other bismuth compounds.
Solubility
Soluble in dilute nitric acid solution, dilute acetic acid, glycerol and acetone. Insoluble in alcohol, ethyl acetateSoluble in acetone, acetic acid and glycerol. Insoluble in ethanol and ethyl acetate.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong acids, strong reducing agents, strong oxidizing agents, powdered metals and organic materials.
Bismuth(III) nitrate pentahydrate is an oxidant for a variety of 4-substituted Hantzsch 1,4-dihydropyridines. It also serves as a reagent for selective oxidation of sufides to sulfoxides. Further, it is involved in the preparation of Dragendorff reagent and used as a TLC stain. It is also used to prepare other bismuth compounds.
Solubility
Soluble in dilute nitric acid solution, dilute acetic acid, glycerol and acetone. Insoluble in alcohol, ethyl acetateSoluble in acetone, acetic acid and glycerol. Insoluble in ethanol and ethyl acetate.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong acids, strong reducing agents, strong oxidizing agents, powdered metals and organic materials.
RUO – Research Use Only
General References:
- Useful, easily handled oxidizing and nitrating agent in organic synthesis. Montmorillonite KSF acidic clay impregnated with bismuth nitrate was found to be an excellent reagent for high-yield aromatic nitration: Tetrahedron Lett., 41, 8017 (2000). Phenols can be nitrated with the salt itself in acetone, or without solvent: J. Org. Chem., 70, 9071 (2005). In the presence of montmorillonite, alcohols can be oxidized to carbonyl compounds: Synth. Commun., 31, 2691 (2001). Reagent for deprotection of oximes: Synthesis, 1010 (2001), and hydrazones: Synth. Commun., 32, 1917 (2001). Thioamides and thioureas are readily converted to their oxo-analogs: Tetrahedron Lett., 44, 591 (2003).
- Catalyst for a convenient, high-yield Michael addition of various substrates, including amines, imidazoles and indoles, to enones: J. Org. Chem., 68, 2109 (2003). Efficient catayst for tetrahydropyranylation and depyranylation of alcohols: Eur. J. Org. Chem., 4891 (2005).
- For a brief feature on uses of the reagent in organic synthesis, see: Synlett, 2699 (2005).
- Zahedifar, M.; Sheibani, H. Rapid three-component synthesis of pyrimidine and pyrimidinone derivatives in the presence of Bi(NO3)3·5H2O as a mild and highly efficient catalyst. Res. Chem. Intermed. 2015, 41 (1), 105-111.
- Pei, C. C.; Leung, W. W. F. Photocatalytic oxidation of nitrogen monoxide and o-xylene by TiO2/ZnO/Bi2O3 nanofibers: Optimization, kinetic modeling and mechanisms. Appl. Catal., B 2015, 174, 515-525.