Search Thermo Fisher Scientific
Thermo Scientific Chemicals
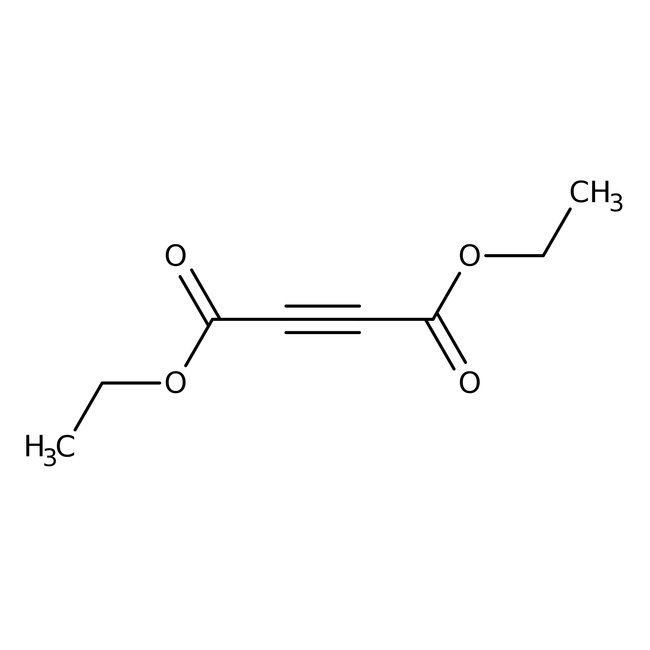
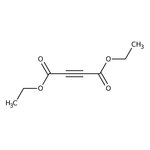
Diethyl acetylenedicarboxylate, 96%, Thermo Scientific Chemicals
CAS: 762-21-0 | C8H10O4 | 170.164 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11719.22 | 100 g |
Catalog number ALFA11719.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS2465-27-2
IUPAC Namehydrogen 4-[4-(dimethylamino)benzenecarboximidoyl]-N,N-dimethylaniline chloride
Molecular FormulaC17H22ClN3
InChI KeyKSCQDDRPFHTIRL-UHFFFAOYSA-N
SMILES[H+].[Cl-].CN(C)C1=CC=C(C=C1)C(=N)C1=CC=C(C=C1)N(C)C
View more
Specifications Specification Sheet
Specification Sheet
Assay (unspecified)Solubility: Soluble in alcohol & slightly soluble in water
FormYellow powder
Assay (Dye)Dye content: ≥ 80 %
Diethyl acetylenedicarboxylate is used as a protein cross-linker. It is also used in the synthesis of 3,4,5-trisubstituted 2(5H)-furanone derivatives, highly functionalized thiazolidinone derivatives, novel cyclic peroxide glucosides and 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethyl acetylenedicarboxylate is used as a protein cross-linker. It is also used in the synthesis of 3,4,5-trisubstituted 2(5H)-furanone derivatives, highly functionalized thiazolidinone derivatives, novel cyclic peroxide glucosides and 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction.
Solubility
Soluble in ethanol, ethyl ether, CCl4, Insoluble in water.
Notes
Store at 4°C. Protect from heat. Store away from oxidizing agents. Incompatible with strong acids and strong bases.
Diethyl acetylenedicarboxylate is used as a protein cross-linker. It is also used in the synthesis of 3,4,5-trisubstituted 2(5H)-furanone derivatives, highly functionalized thiazolidinone derivatives, novel cyclic peroxide glucosides and 4,11-dimesitylbisanthene, soluble bisanthene derivative, via Diels-Alder reaction.
Solubility
Soluble in ethanol, ethyl ether, CCl4, Insoluble in water.
Notes
Store at 4°C. Protect from heat. Store away from oxidizing agents. Incompatible with strong acids and strong bases.
RUO – Research Use Only
General References:
- YW Guo; YL Shi; HB Li; M Shi. Reactions of salicyl N-tosylimines or salicylaldehydes with diethyl acetylenedicarboxylate for the synthesis of highly functionalized chromenes.Tetrahedron. 2006, 16 5875-5882.
- Alan R. Katritzky; Jiangchao Yao; Weiliang Bao; Ming Qi; and Peter J. Steel. 2-Benzotriazolylaziridines and Their Reactions with Diethyl Acetylenedicarboxylate.J. Org. Chem. 1999, 64 (2), 346-350.
- For reactions of acetylenedicarboxylic esters, see Dimethyl acetyl enedicarboxyl ate, A11437.
- Reaction with enamines leads to 2(1H)-pyridones: Synthesis, 371 (1992).